2687
Short T2* quantification of knee structures based on accelerated UTE Spiral VIBE MRI with SPIRiT reconstruction1Siemens Healthcare NV/SA, Beersel, Belgium, 2Department of Radiology, Antwerp University Hospital and University of Antwerp, Edegem, Belgium, 3imec-Vision Lab, Department of Physics, University of Antwerp, Wilrijk, Belgium
Synopsis
Clinical validation of quantitative UTE MRI techniques in musculoskeletal studies remains limited, despite their potential to unveil short T2* information. As these techniques require considerably more scan time than conventional imaging, there is a need for integration of acceleration methods. This study investigated the use of the UTE Spiral VIBE sequence with in-plane acceleration and SPIRiT reconstruction for short T2* mapping of knee structures. As similar image quality and T2* values were obtained for the non-accelerated and accelerated acquisitions, the UTE Spiral VIBE technique shows great promise for fast UTE T2* MRI of the knee.
Introduction
Densely packed collagen-based knee structures, such as ligaments and tendons, are characterized by an intrinsically short T2* relaxation, resulting in very low signal intensity on conventional MRI. To visualize these tissues and their internal structure, ultrashort echo time (UTE) MRI needs to be employed as this allows to sample the rapidly decaying signals before they reach steady state. By acquiring multiple echoes, these sequences enable the quantification of short (1–10 ms) and ultrashort (0.1–1.0 ms) T2* relaxation times.1 UTE MRI thus has the potential to quantify changes in composition and structure that could be indicative of tissue pathologies such as meniscal degeneration and osteoarthritis,2 or tissue maturation after the replacement or repair of injured anterior cruciate ligaments.3A variety of UTE T2* mapping sequences differing in their k-space sampling pattern (e.g. radial, stack-of-spirals and 3D cones) have been developed, yet their clinical validation remains limited as they generally require significantly more scan time than conventional musculoskeletal (MSK) sequences.1 However, by incorporating acceleration techniques into the existing sequences, quantitative UTE MRI could become more clinically attractive.
The aim of this study was to evaluate the feasibility of using the UTE Spiral VIBE sequence with in-plane acceleration and SPIRiT reconstruction to quantify short T2* values of knee structures within a clinically acceptable scan time, by drawing a qualitative and quantitative comparison with the non-accelerated acquisition.
Methods
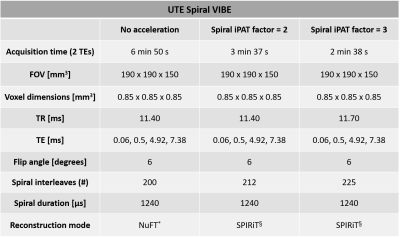
A female volunteer (age: 25 years, left knee) with no known prior or current knee injuries was scanned on a 3T MR scanner (MAGNETOM Prisma Fit, Siemens Healthcare, Erlangen, Germany) with a 15-channel knee coil (QED, Mayfield Village, OH, United States). All images were acquired with a prototypical UTE Spiral VIBE. This 3D UTE sequence uses a stack-of-spirals k-space trajectory with adaptive echo time and allows for in-plane acceleration by undersampling outer regions of the k-space.4,5First, a non-accelerated version was acquired with parameters as in Kim et al. (Table 1).6 Subsequently, the spiral iPAT factor (i.e. the in-plane acceleration factor) was set to 2 for the second set of acquisitions, and to 3 for the third set of acquisitions. In both cases, the reconstruction mode was changed to the iterative parallel image reconstruction SPIRiT (Table 1).7 For each of the three implementations, fat suppression was applied and 4 TEs were collected with 2 acquisitions per implementation. All datasets were registered to their first echo image through diffeomorphic mapping with a symmetric normalization model in ANTs.8 After image registration, T2* mapping was performed by voxel-wise fitting of a mono-exponential T2* relaxation model to the data. To determine the T2* mean and standard deviation values of selected knee structures, volumes-of-interest (VOIs) were defined on the computed difference images (TE1 – TE3) in the following structures: anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), patellar tendon, quadriceps tendon, medial and lateral menisci, patellar cartilage and lateral gastrocnemius muscle. All VOIs were verified by an MSK radiologist. Differences between the calculated T2* values of the non-accelerated implementation and the accelerated ones were evaluated for every structure.
Results
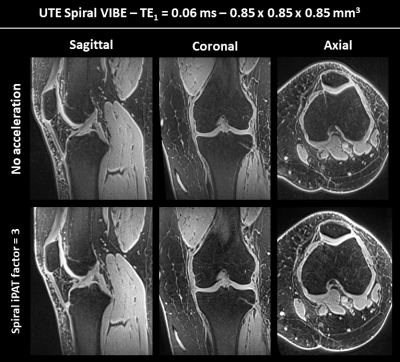
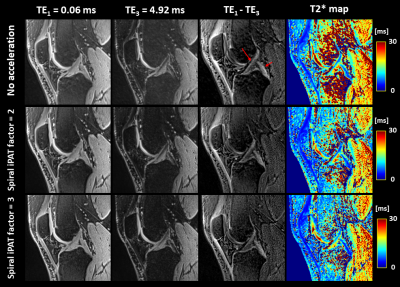
Figure 1 shows a reconstructed slice of the isotropic first echo (TE1) for each of the three main anatomical directions. When comparing the images of the first row (no acceleration) and second row (spiral iPAT factor of 3), no considerable differences can be observed in terms of image quality.Figure 2 displays the first and third echo image of a selected sagittal slice, as well as their difference image, for all datasets. The difference images are included to highlight the short T2* structures, such as the ACL (long red arrow) and PCL (short red arrow). The right-most column depicts the computed T2* maps and corresponding color bars.
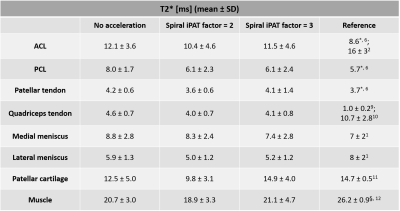
Table 2 provides an overview of the mean T2* values and the standard deviations (SD) computed for each of the 8 VOIs in the non-accelerated and accelerated datasets. T2* reference values from recent UTE literature are provided in the right-most column.
Discussion
Acceleration of spiral UTE MRI with SPIRiT reconstruction allows to reduce the total scan time by a factor of up to 2.5 and does not seem to deteriorate the image quality as shown in Figures 1 and 2. Note that T2* differences in bone and fat regions result from fitting errors due the use of fat suppression. The denoising characteristics of SPIRiT could explain the lower values for the accelerated datasets.Results listed in Table 2 suggest that in-plane acceleration and subsequent reconstruction do not considerably affect the T2* quantification. Similar T2* values can be observed across all datasets for each of the evaluated knee structures. Computed T2* values are also in line with short T2* values reported in recent UTE literature.
Conclusion
The UTE Spiral VIBE sequence shows great promise for fast UTE T2* MRI of the knee as it provides good image quality and robust T2* mapping of knee structures for in-plane acceleration factors of up to 3, which results in considerably shorter scan times. Future work will focus on fine-tuning the accelerated acquisition to reduce vague reconstruction artifacts, and on further validating the sequence in human subjects to evaluate its clinical applicability.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 764513. The authors would also like to thank Thomas Benkert and Josef Pfeuffer from Siemens Healthcare for their technical support.References
1. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J. Magn. Reson. Imaging. 2015;41(4):870-883.
2. Williams A, Qian Y, Golla S, et al. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20(6):486-494.
3. Chu C, Williams A. Quantitative MRI UTE-T2* and T2* show progressive and continued graft maturation over 2 years in human patients after anterior cruciate ligament reconstruction. OJSM. 2019;7(8).
4. Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn. Reson. Med. 2008;60:135-145.
5. Mugler JP, Fielden SW, Meyer CH, et al. Breath-hold UTE lung imaging using a stack-of-spirals acquisition. Proc. Intl. Soc. Mag. Reson. Med. 2015;23(1476).
6. Kim J, Lartey R, Liu K, et al. Comparison of radial and spiral UTE MRI and T2* quantification of the knee joint. Proc. Intl. Soc. Mag. Reson. Med. 2019;27(1393).
7. Lustig M, Pauly JM. SPIRiT: iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn. Reson. Med. 2010;64:457-471.
8. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
9. Krämer M, Maggioni MB, Brisson NM, et al. T1 and T2* mapping of the human quadriceps and patellar tendons using ultra-short echo-time (UTE) imaging and bivariate relaxation parameter-based volumetric visualization. Magn. Reson. Imaging. 2019;24(63):29-36.
10. Wu M, Zhao W, Lee J, et al. A protocol for comprehensive quantitative 3D Ultrashort Echo Time (UTE) cones MR imaging of the knee joint with motion correction. Proc. Intl. Soc. Mag. Reson. Med. 2019;27(4387).
11. Qian Y, Williams A, Chu C, et al. Repeatability of UTE-based two-component T2* measurements on cartilages in human knee at 3T. Magn. Reson. Med. 2013;69(6):1564-1571.
12. Vandenborne K, Walter G, Ploutz-Snyder L, et al. Relationship between muscle T2* relaxation properties and metabolic state: a combined localized 31P-spectroscopy and 1H-imaging study. Eur. J. Appl. Physiol. 2000;82(1-2):76-82.
Figures