2647
Retrospective affine motion correction of arterial spin labeling kidney perfusion measurements
Steffen Ringgaard1 and Anne Dorte Blankholm2
1MR Research Centre, Aarhus University, Aarhus, Denmark, 2Department of Radiology, Aarhus University Hospital, Aarhus, Denmark
1MR Research Centre, Aarhus University, Aarhus, Denmark, 2Department of Radiology, Aarhus University Hospital, Aarhus, Denmark
Synopsis
When doing arterial spin labeling perfusion measurements in the kidneys, respiratory motion is an important issue. We compared different methods for doing retrospective motion correction of single-shot ASL images. We evaluated the temporal signal variation in the cortex after correction by translation, translation+rotation and affine transformation and found that the variation was lowest when using full affine correction. Besides, we also compared different similarity measures as used during the motion correction.
Introduction
Measurement of blood perfusion in the kidneys is important for diagnosing kidney diseases, and arterial spin labeling (ASL) is preferable over contrast agent based methods. Measurement can be performed with either the FAIR or pCASL methods, and for both methods, quantitative perfusion maps is based on subtraction of label and control images. For obtaining sufficient signal-to-noise ratio, a number of repetitions are needed, because the signal difference between label and control images is only 4-6 % of the equilibrium magnetization. Usually 20-30 repetitions are applied.Due to respiration, the kidneys are moving several centimeters, mainly in foot-head direction. Usually prospective motion correction (such as gating to a respiration sensor) is applied, but still severe motion of the images is seen. The perfusion is mainly in the renal cortex, which is only 3-6 mm thick, and for obtaining reliable perfusion values, careful retrospective motion correction is therefore necessary. This can be done in various ways and both manual correction1, rigid body motion correction2 and correction using affine transformation3 of the kidneys has been applied.
We have performed a systematic analysis of the effect of various motion corrections on the robustness of the resulting perfusion maps.
Methods
Kidney perfusion was assessed in 19 healthy volunteers using a 1.5T Philips Achieva dStream MR scanner. Images were acquired in an oblique coronal orientation using FAIR labelling and single-shot spin echo data sampling. Five slices were sampled with a slice thickness of 5 mm and 3x3 mm in-plane resolution. Twenty sets of label and control images were acquired over 7 minutes. Respiration gating was used with a respiration sensor placed on the abdomen.Motion correction was made using affine transformation of the kidneys and similarity with the first control image was obtained for the various affine transformations. Different sets of transformations were investigated:
- No retrospective correction.
- Correction by combined rigid body translation for both kidneys.
- Correction by rigid body translation separately for each kidney.
- Correction by individual rigid body translation + rotation.
- Full affine individual transformation. This included translation, rotation, scaling and shearing (Figure 1).
- Mean absolute pixel difference.
- Mean squared pixel difference.
- Mean absolute pixel difference of image gradient maps.
The accuracy of the various motion corrections was assessed by segmenting out the cortex (6 mm rim in the kidney edges) in the reference image. The standard deviation of pixel intensities over the 20 repetitions was calculated for each correction method. This was used as a measure of noise in the calculated perfusion maps.
Results
The standard deviation of the signal in the cortex through the 20 dynamics can be seen in Figure 2. There was a statistically significant difference using global translation and no correction (p<0.001), between individual rigid body motion correction for each kidney and global translation (p<0.001), and between full affine correction and rigid body correction (translation+rotation) (p<0.001). No difference was found between individual kidney translation and individual kidney translation+rotation.Comparing the three different similarity measures we found that using absolute difference performed slightly better than using the squared difference and also than using absolute difference of gradient maps (Figure 3).
By proper motion correction, clearer perfusion maps can be generated. In Figure 4, an example of perfusion maps generated by combined kidney rigid body motion correction and full affine corrected are compared.
Discussion
The main conclusions from the study are that retrospective motion correction of ASL perfusion measurement is necessary prior to quantitation of perfusion. Significant improved correction is obtained by individual correcting each kidney, indicating that the two kidneys are displaced differently by respiration. A further improvement was found, when full affine correction was used.The study also showed that the most efficient way to assess the similarity of two kidneys as applied during the correction process, is to use either the absolute difference or squared difference and not use gradient maps.
The main limitation of this work is that we did only in-plane motion correction and did not take through-plane motion into account. This was partially because the motion in the through-plane direction was small using the oblique-coronal slice orientation, and partially because the slice thickness was larger (5 mm) than the pixel size. To obtain better motion correction, 3D imaging with retrospective correction in all 3 directions might be used, but this is difficult to do with single-shot imaging, and with multi-shot imaging the motion artifacts are much more difficult to handle.
Without careful motion correction, the edges of the kidneys can be enhanced in the perfusion maps due to the motion, and because the blood perfusion is mainly in the cortex, this might be confused with high perfusion values.
During development of the methods, we also tried to use non-affine correction, but this was not robust enough, and we continued with the affine correction method.
Conclusion
Retrospective motion correction is necessary for kidney ASL measurement, and full affine correction was optimal.Acknowledgements
NoneReferences
- Buchanan CE, Cox EF, Francis ST. Evaluation of 2D imaging schemes for pulsed arterial spin labeling of the human kidney cortex. Diagnostics 2018;8:43.
- Artz NS, Sadowski EA, Wentland AL, et al. Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging 2011;29:74-82.
- Gardener AG, Francis ST. Multislice perfusion of the kidneys using parallel imaging: Image acquisition and analysis strategies. Magn Reson Med. 2010;63:1627–1636.
Figures
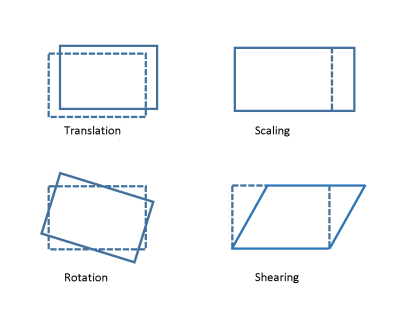
Figure 1. Modes of
affine transformations.
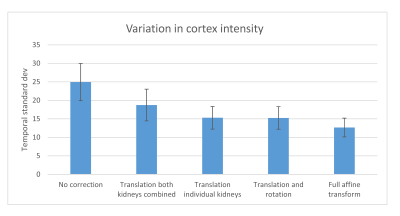
Figure 2. Temporal
standard deviation of signal in the kidney cortex after different motion
correction methods. Using full affine transform gives lowest noise in the
perfusion maps.
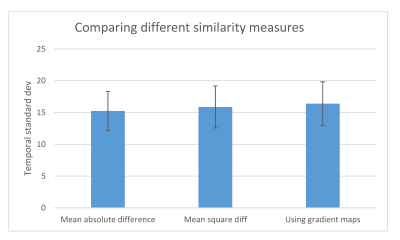
Figure 3. Comparison
of different similarity measures used in the affine motion correction. Using absolute
difference gives lowest noise.
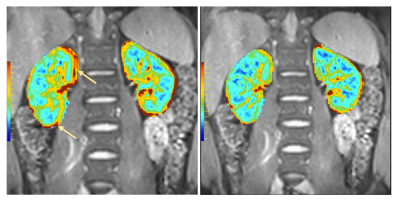
Figure 4. Perfusion
maps. Left: using global motion correction, right: using full affine correction.
Arrows indicates regions with erroneous perfusion values due to motion.