2625
Feasibility of 4D-Flow MRI of the Liver using Pencil Beam Navigator for Respiratory Gating and Compressed Sensing1Department of Medical and Health Sciences, and Center for Medical Image Science and Visualization, Linköping University, Linköping, Sweden, 2Siemens Healthcare AB, Malmö, Sweden, 3Siemens Medical Solutions USA, Inc, Cleveland, OH, United States
Synopsis
Four-dimensional flow magnetic resonance imaging of the liver can be used to characterize the blood flow in the portal vein. This could be a useful tool to evaluate portal hypertension. However, four-dimensional flow magnetic resonance imaging typically requires a long acquisition time, which limits clinical usefulness. We therefore investigated if compressed sensing can be used to shorten the acquisition time. We found that the acquisition time could be reduced to 8-10 minutes, without impacting the flow measurements.
Purpose
Portal hypertension is clinically highly significant and early signs are therefore of importance. Quantitative measurement of hepatic blood flow would be useful to the understanding of disease progression in the cirrhotic liver, particularly the early development of fibrosis and inflammation [1], since the development of liver fibrosis and lipid accumulation constrict the blood flow to the liver.Four-dimensional flow (4D-Flow) magnetic resonance imaging (MRI) is an emerging method for quantitative evaluation of hemodynamics of in the liver and abdomen, however, it is limited to be used in clinical setting due to the long acquisition time. Compressed sensing (CS) is a method for image acquisition acceleration that is gaining in popularity in abdominal imaging. However, to the best of our knowledge, it has not been applied yet to abdominal 4D-Flow.
Our aim here was therefore to evaluate the feasibility of using compressed sensing to accelerate image acquisition in liver 4D-Flow MRI.
Subjects and Methods
Two healthy volunteers (aged 28 and 33 years) were included in this study. They were instructed to fast for three hours. The local ethics committee approved this study, and written informed consent was obtained from healthy volunteer.4D-Flow MRI with CS [2] and with conventional GRAPPA and CS with acceleration factor R = 7.6 and 3, respectively, was performed using prototype sequences on a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany). 4D-Flow measurements were performed with respiratory gating using with a pencil beam navigator placed on the liver dome with Respiratory Controlled Adaptive k‐space Reordering (ReCAR) to re-duce respiratory motion artifacts, ECG retrospective gating and with the following sequence parameters: isotropic voxel resolution 2.68 mm3, velocity encoding 50 cm/s and TR/TE/flip angle: 46.5 ms/3.3 ms/7°. CS 4D-Flow was acquired twice to investigate the repeatability of this new measurement technique.
Analysis of the 4D-Flow data was performed in GTFlow (GyroTools LLC, Zürich, Switzerland). Eddy currents were corrected for by linear phase correction, which was done automatically within the software. Peak velocity and net flow were estimated from a region of interest of the portal vein, below the main branching.
Results
In Table 1 are summarized the peak velocity, net flow, total acquisition time and mean RR time interval for all three 4D-Flow measurements and for both volunteers. The mean RR time interval was very similar across the three 4D-Flow measurements.Similar peak velocities and net flows between the three 4D-Flow experiments are reported for each volunteer. The CS 4D-Flow acquisition was performed within a third of the total scan time to the non-CS 4D-Flow scan. CS 4D-Flow showed to provide similar acquisition times and flow parameters in the repeatability experiment.
Fig. 1 shows the net portal blood flow over a cardiac cycle with an overall good agreement.
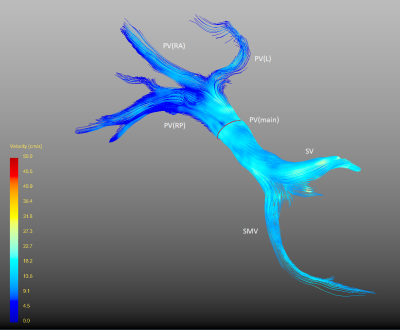
Fig. 2 visualizes color-coded streamlines with respect to velocities the in the por-tal venous system. The figure indicates blood flow can be visualized and meas-ured in both anterior and posterior right branch, as well as the left branch of the portal vein.
Discussion
The difference between conventional and CS 4D-Flow seen in the flow curves (Fig. 1) could be related to a not-optimized number of CS-iterations and regularization parameters for the small velocities (cf. VENC 50) present in the portal vein.A very reasonable repeatability of the CS measurements with respect to both scan time and derived flow parameters is shown in Table 1. The faster CS 4D-Flow acquisition is thus not related to variation of mean of the RR interval time and the acceptance rate of the respiratory navigator was similar in between measurements.
The possibility to use the pencil beam navigator for respiratory gating opened the possibility to place the navigator such to gate directly on the liver-lung inter-face,i.e. the organ of interest, instead of placing the crossed-pair respiratory navigator on another lung interface to track the respiratory motion, e.g. at the spleen-lung interface as in [3]. A visual inspection did not show any effect of the pencil beam navigator on the velocity encoded data and provided good respiratory gating.
Our main result was that it was possible to reduce acquisition time significantly by using compressed sensing, in combination with the pencil beam navigator. The results agreed very well with both conventional 4D-Flow (Table 1).
Conclusion
We present in this abstract preliminary result of first acquisitions of 4D-Flow MRI in the liver using CS as an acquisition acceleration technique and pencil beam for respiratory navigation placed on the liver dome. A good overall agreement was obtained between GRAPPA and CS accelerated 4D-Flow MRI. CS 4D-Flow MRI was performed within a clinical acceptable measurement time of 8-10 minutes and showed good repeatability, both in terms of acquisition time and derived parameters. Additional volunteers need to be included to improve the statistical analysis and flow parameters derived from 4D-Flow should be compared against values from 2D-Flow measurements to validate 4D-Flow MRI in the liver.Acknowledgements
GyroTools is gratefully acknowledged for providing a test license.
The Science Research Council is acknowledged for supporting this work by a grant to PL
References
1. Stankovic, Z. World J Gastroenterol, 2016. 22(1) 89-102.
2. Ma, L. Magn Reson Med. 2019. 81 3675–3690.
3. Stankovic, Z. J Magn Reson Imaging. 2010. 32 466–475.
Figures