2622
An Inline Implementation of Free Breathing 2D Radial MRE with Radial Self Gating
Bradley Bolster, Jr.1, Stephan Kannengiesser2, and Vibhas Deshpande3
1Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Medical Solutions USA, Inc., Austin, TX, United States
1Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Medical Solutions USA, Inc., Austin, TX, United States
Synopsis
This study demonstrates in inline implementation of a radial free breathing MRE sequence with integrating radial self gating. Measured stiffness values are consistent across self-gating threshold values and results are in good agreement with standard breath-hold MRE techniques.
Introduction
While most adults can reliably perform the multiple breath holds required for the modern abdominal MRI exams, this is not typically the case for children or the very sick. Eliminating breath holds from as many protocols as possible increases the accessibility of these diagnostic tools to a patient population which can benefit substantially from them. Magnetic Resonance Elastography (MRE) provides a non-invasive method of assessing hepatic stiffness and liver fibrosis1. Because this technique is sensitive to very small oscillating displacements in the tissue, it is potentially challenging to base it on free-breathing acquisitions. Previously, radial k-space trajectories have been applied to MRE along with self-gating to improve accuracy of stiffness measurements in the presence of respiratory motion2. In this study we present a 2D gradient echo (GRE) based radial free breathing MRE sequence with radial self-navigation implemented inline. The impact of this motion mitigation technique is evaluated in normal volunteers at both 1.5 and 3T.Methods
Sequence: A prototype 2D radial GRE based MRE sequence was implemented. Linear interleaved radial view ordering was used in this validation which divided the total number of views into 8 interleaves. MRE motion encoding was through plane using a full cycle, first moment nulled, motion encoding gradient (MEG). Two MEG polarities were acquired for each view. A full set of radial k-space was acquired at each polarity for each of four different phase offsets by augmenting the trigger for the MRE mechanical driver (Resoundant, Rochester MN, USA) which was operating at 60Hz. Reconstruction Pipeline: Acquired views were processed to extract the self-gating signal from the k-space center samples at a temporal resolution of 2*TR = 100ms from each receive coil channel. The resulting waveforms were baseline-corrected and the channel with the best signal quality and consistency across all phase offsets was selected as the input to the self-gating binning algorithm. A representative self-gating waveform is shown in Figure 1. Based on a predefined percentage this algorithm binned the views occurring at or near end expiration determined as the values at which the self-gating signal spent the most time. The reduced view set was then regridded for each MEG polarity and processed with the standard 2D MRE inversion. Seven free-breathing datasets were collected in three healthy volunteers. Acquisitions were performed at both 1.5 and 3T (MAGNETOM Aera (6) and MAGNETOM Prismafit(1), respectively; Siemens Healthcare, Erlangen, Germany). Each subject underwent informed consent under IRB oversight. A Cartesian breath-held (BH) GRE MRE acquisition was performed on each volunteer followed by one or more free-breathing (FB) radial MRE acquisitions using the prototype sequence. Table 1 shows the range of imaging parameters applied for both the BH and FB sequences. Raw data were saved to enable retrospective reconstructions with different parameter settings. Data from each subject was reprocessed by the inline reconstruction implementation for a variety of self-gating thresholds ranging from 20% of the data kept to the full 100%. The resulting image data and stiffness maps were analyzed using a custom MATLAB script (Mathworks, Inc., MA, USA) that defined a region of interest (ROI) inside a manual segmentation of the liver. Voxels with > 90% CI (as calculated by the inversion) were included in that ROI. Mean and standard deviation of stiffness in the ROI as well as the number of included pixels were calculated in each case.Results
The addition of self-gating to the reconstruction pipeline resulted in a negligible increase in reconstruction time. In the single slice acquisitions collected in this study all reconstructions completed less than 30 seconds after the end of the scan. The algorithm was easily configurable from the scanner UI. Magnitude, stiffness and wave images with 90% CI contours for a representative dataset are shown in Figure 2. As shown in the figure stiffness values of the radial self-gated acquisition are within 2% of the standard BH gradient echo acquisition. Figure 3 shows a plot of stiffness difference between the FB-radial acquisition and the BH-cartesian acquisition as a function of radial self-gating percentage for the 7 datasets acquired for this study. Except for a few low signal-to-noise outliers, calculated stiffness values within the 90% CI ROI were consistent across all self-gating percentages. In addition, these values compared favorably with conventional Cartesian breath-hold MRE. Though not shown here, ROI area trended higher with increasing self-gating percentage, presumably as a function of increased signal-to-noise ratio (SNR) with more views being used in the reconstruction.Conclusion
We have presented an inline implementation of radial free breathing MRE with radial self-gating integrated into the reconstruction pipeline. This study had two primary findings: measured stiffness very little with self-gating threshold and the stiffness values using the FB-MRE sequence are in strong agreement with traditional Cartesian BH MRE. These findings are consistent with published results using manual offline versions of this technique. The inline implementation demonstrated in this study will enable a larger-scale in-vivo validation going forward. Further refinement of self-gating acquisition and waveform definition techniques may increase the impact of self-gating over uncorrected FB acquisitions.Acknowledgements
No acknowledgement found.References
1. Venkatesh, Sudhakar K., Meng Yin, and Richard L. Ehman. "Magnetic resonance elastography of liver: technique, analysis, and clinical applications." Journal of magnetic resonance imaging 37.3 (2013): 544-555.
2. Holtrop, Joseph et al. “Free Breathing Radial Magnetic Resonance Elastography” Proc. Int. Soc. Magn. Reson. Med. 27th. p 4482 (2019).
Figures
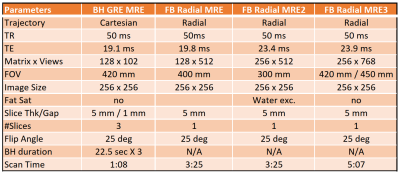
Table 1: Scan parameters for acquisitions in this study
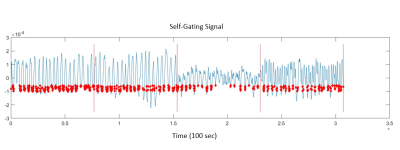
Figure 1:
A representative self-gating signal.
Red dots reflect the views reconstructed for a 20% gating threshold.
Red lines represent the division between
different phase offsets. All phase
offsets are evaluated as a single bin.
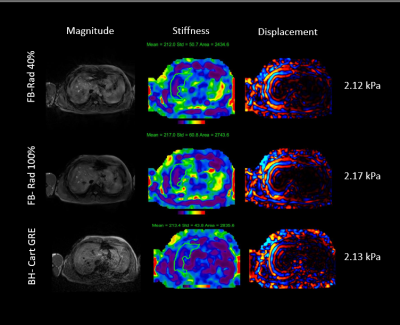
Figure 2: Representative images from the Radial Free Breathing MRE sequence as compared to standard Cartesian GRE MRE. ROIs shown were added in the offline data analysis for this study.
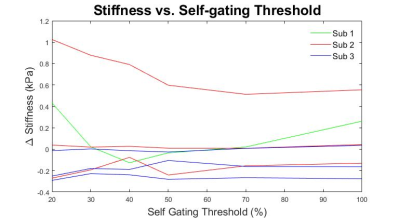
Figure 3: Stiffness differences between radial FB-MRE and standard Cartesian BH-MRE as a function of self gating threshold. Two acquisitions had very low SNR and show up as outliers in this plot.