2605
Correlation between CT based Radiomics Features and Mesenteric Vein Resection Margin in Patients with the Pancreatic Head Cancer1Radiology, Changhai hospital, Shanghai, China, 2Pancreatic Surgery, Changhai hospital, Shanghai, China, 3Shanghai Institute for Advanced Communication and Data Science, School of Computer Engineering and Science, Shanghai University, Shanghai, China
Synopsis
A striking number of patients are diagnosed with resectable pancreatic cancer (PC) or borderline resectable PC by computed tomography (CT) but end up with positive (R1) resection at surgery according to the National Comprehensive Cancer Network (NCCN) criteria. If the pathological resection margin can be accurately and noninvasively predicted prior to surgery, an appropriate treatment plan can be developed and patients with PC can avoid futile surgery. Hence, we sought to accurately identify the relationship between the arterial radiomics score (rad-score) and pathologic superior mesenteric vein (SMV) resection margin in patients with pancreatic head cancer and to evaluate the diagnostic performance of the rad-score in differentiating between negative (R0) and R1 resection.
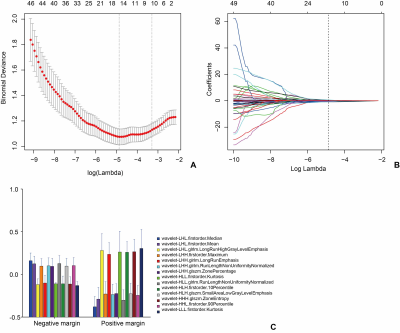
Materials and Methods A total of 181 patients with pathologically confirmed cancer in the head of the pancreas who underwent multislice computed tomography within one month of resection between January 2016 and December 2018 were retrospectively investigated. For each patient, 1029 radiomics features of the arterial phase were extracted, which were reduced using the least absolute shrinkage and selection operator (LASSO) logistic regression algorithm. Multivariate logistic regression models were used to analyze the association between the arterial rad-score and SMV resection margin. The arterial rad-score performance was determined by its discrimination and clinical usefulness.
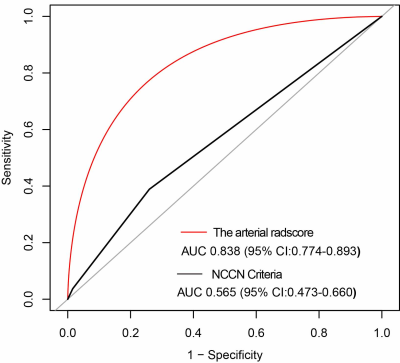
Results The arterial rad-score is based on 14 selected arterial phase features by LASSO logistic regression algorithm. Multivariate analyses confirmed a significant and independent association between the arterial rad-score and SMV resection margin (P<0.0001). The arterial rad-score were in its high accuracy (AUC=0.8380). The best cut point based on maximizing the sum of sensitivity and specificity was -0.7108. DCA demonstrated that the radiomics nomogram was clinically useful.
Conclusions The arterial rad-score, is a potentially valuable noninvasive tool for accurate preoperative prediction of the SMV resection margin.
Acknowledgements
National Science Foundation for Scientists of China (81871352), National Science Foundation for Young Scientists of China (81701689, 81601468), 63-class General Financial Grant from the China Postdoctoral Science Foundation (2018M633714), Key Junior College of National Clinical of China, Shanghai Technology Innovation Project 2017 on Clinical Medicine (17411952200), Project of Precision Medical Transformation Application of NMMU (2017JZ42), and Top Project of the Military Medical Science and Technology Youth Training Program (17QNP017).References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73-85.
3. De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89(8):626-32.
4. Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg. 2013;257(4):731-6.
5. Rau BM, Moritz K, Schuschan S, et al. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. 2012;152(3 Suppl 1):S103-11.
6. Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):202-10.
7. Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17(5.5):603-5.
8. Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22(4):1153-9.
9. Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10(10):1338-45; discussion 45-6.
10. Noda Y, Goshima S, Kawada H, et al. Modified National Comprehensive Cancer Network Criteria for Assessing Resectability of Pancreatic Ductal Adenocarcinoma. AJR Am J Roentgenol. 2018;210(6):1252-8.
11. Fang CH, Zhu W, Wang H, et al. A new approach for evaluating the resectability of pancreatic and periampullary neoplasms. Pancreatology. 2012;12(4):364-71.
12. Garces-Descovich A, Beker K, Jaramillo-Cardoso A, et al. Applicability of current NCCN Guidelines for pancreatic adenocarcinoma resectability: analysis and pitfalls. Abdom Radiol (NY). 2018;43(2):314-22.
13. Tamburrino D, Riviere D, Yaghoobi M, et al. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016;9:CD011515.
14. Hong SB, Lee SS, Kim JH, et al. Pancreatic Cancer CT: Prediction of Resectability according to NCCN Criteria. Radiology. 2018;289(3):710-8.
15. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441-6.
16. Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234-48.
17. Verbeke FCS. Pathology of the Pancreas: A Practical Approach. London: Springer; 2013.
18. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging manual. 8 ed. New York: Springer; 2017.
19. Campbell F, Verbeke CS. Pathology of the Pancreas. London: Springer; 2013.
20. Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009;36(13):2495-501.
21. Kitagawa H, Ohta T, Makino I, et al. Carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Front Biosci. 2008;13:2728-35.
22. Makino I, Kitagawa H, Ohta T, et al. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas. 2008;37(4):358-65.
23. van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77(21):e104-e7.
24. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective J. R. Statist. Soc. B (2011). 2011;73:273–82.
25. Gadducci A, Cavazzana A, Cosio S, et al. Lymph-vascular space involvement and outer one-third myometrial invasion are strong predictors of distant haematogeneous failures in patients with stage I-II endometrioid-type endometrial cancer. Anticancer Res. 2009;29(5):1715-20.
26. Dekker TJ, van de Velde CJ, van Bruggen D, et al. Quantitative assessment of lymph vascular space invasion (LVSI) provides important prognostic information in node-negative breast cancer. Ann Oncol. 2013;24(12):2994-8.
27. Briet JM, Hollema H, Reesink N, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96(3):799-804.
28. Liang W, Yang P, Huang R, et al. A Combined Nomogram Model to Preoperatively Predict Histologic Grade in Pancreatic Neuroendocrine Tumors. Clin Cancer Res. 2019;25(2):584-94.
29. Yang J, Guo X, Ou X, et al. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front Oncol. 2019;9:494.
30. Gu D, Hu Y, Ding H, et al. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol. 2019.
31. Kim BR, Kim JH, Ahn SJ, et al. CT prediction of resectability and prognosis in patients with pancreatic ductal adenocarcinoma after neoadjuvant treatment using image findings and texture analysis. Eur Radiol. 2019;29(1):362-72.
32. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BJOG. 2015;122(3):434-43.
33. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-74.
Figures