2604
The feasibility of model-free machine learning with variable features of MRI images on the prostate cancer application1Department of Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, 2Health system, Philips, Taipei, Taiwan, 3Healthcare, Philips, Hong Kong, China, 4Department of Medical Imaging, Tungs’ Taichung Metroharbor Hospital, Taichung, Taiwan
Synopsis
Several advanced post-processing methods were used to assess prostate cancer, including diffusion model of IVIM, T2 mapping with multi-echo turbo spin echo , and permeability analysis with Tofts model. We found that it is not easy to overcome the fitting error, and it is not reasonable to directly integrate IVIM, T2 mapping and permeability together as well. The aim of this study is to evaluate the feasibility of the prostate cancer screening with model-free machine leaning , and to test different combinations of input data, including multiple b-value diffusion weighted imaging (DWI), multi-echo T2w TSE, and dynamic contrast enhanced (DCE) images.
Introduction
Magnetic resonance imaging (MRI) plays an important role in the diagnosis of prostate carcinoma, and multi-parametric MRI is introduced to improve the accuracy of the detection of prostate cancer1. Several advanced post-processing methods were used to assess prostate cancer, including diffusion model of intravoxel incoherent motion (IVIM), T2 mapping with multi-echo turbo spin echo (TSE), and permeability analysis with Tofts model2-4. We found that it is not easy to overcome the fitting error, ex. IVIM, during the calculation, and it is not reasonable to directly integrate IVIM, T2 mapping and permeability together as well. The aim of this study is to evaluate the feasibility of the prostate cancer screening with model-free machine leaning (ML), and to test different combinations of input data, including multiple b-value diffusion weighted imaging (DWI), multi-echo T2w TSE, and dynamic contrast enhanced (DCE) images.Materials and Methods
Nine patients with prostate cancer were examined with a 3T MRI system (Achieva 3T X-series, Philips Healthcare, Best, The Netherland). All the data were collected after obtaining the approval from a local institutional review board. Multiple b-values DWI (b=0, 50, 100, 200, 400, 600, 1000, 1200, 1800, and 2000), multi-echo T2W TSE (TE=12, 24, 36, 48, 60, 72, 84, and 96 ms), and DCE 3D gradient echo with fat-suppression (4 secs per volume, total 20 volumes) were collected with the same geometry setting. All 4-dimension images were reconstructed in the same matrix size based on acquired MR images. To label the ground truth of central zone (cz), peripheral zone (pz) and tumor, a ROI-drawing tool was applied. Cubic support vector machine (cubicSVM) was used to classify the selected ROIs into three categories (cz, pz and tumor) in this study. There were seven prostate cancer patients’ images for the cubicSVM model training process and two prostate cancer patients’ images for the testing. DCE images, DWI images and multi-echo T2W TSE images were considered as three features for the cubicSVM training phase in this study. Furthermore, different combination of features was evaluated to find the optimal classification model.Results
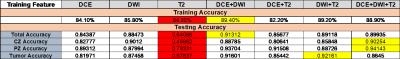
In the training phase, The accuracy for classification of all feature combinations reached over 80 % in the training phase, except for that of the training model with T2W images only (64.3 %). Moreover, in the testing phase, the accuracy also reached approximately 90% in both DCE+DWI model and DCE+DWI+T2 model (Table 1). In Figure 1, MR images with pre-selected prostate ROI were applied to the pre-trained model, and the result showed that tumor has been successfully identified.Discussion and Conclusion
When T2W images were the only feature for classification, the lowest accuracy was observed for classification. However, the DWI+T2 model exhibited the highest accuracy for the feature classification of prostate cancer, which was interestingly consistent with the clinical standard process to identify tumors. In this study, we proposed a model-free method for prostate image classification, which has the potential to be applied in clinical prostate tumor screening.Acknowledgements
No acknowledgement found.References
1. Langer, D.L., et al., Prostate cancer detection with multi-parametric MRI: Logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. 2009. 30(2): p. 327-334.
2. Zhang, Y.-D., et al., The Histogram Analysis of Diffusion-Weighted Intravoxel Incoherent Motion (IVIM) Imaging for Differentiating the Gleason grade of Prostate Cancer. 2015. 25(4): p. 994-1004.
3. Gibbs, P., et al., Comparison of quantitative T2 mapping and diffusion-weighted imaging in the normal and pathologic prostate. 2001. 46(6): p. 1054-1058.
4. Hara, N., et al., Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a useful modality for the precise detection and staging of early prostate cancer. 2005. 62(2): p. 140-147.
Figures