2602
Retrospective Motion Artifact Reduction with CNN (MARC) combined with model-based artifact simulation for T2WI of the liver
Motohide Kawamura1, Daiki Tamada1, Tetsuya Wakayama2, Hiroshi Onishi1, and Utaroh Motosugi1
1Department of Radiology, University of Yamanashi, Yamanashi, Japan, 2MR Collaboration and Development, GE Healthcare, Tokyo, Japan
1Department of Radiology, University of Yamanashi, Yamanashi, Japan, 2MR Collaboration and Development, GE Healthcare, Tokyo, Japan
Synopsis
Motion artifact is a problem in abdominal imaging. Respiratory-triggering techniques are commonly used to suppress the motion artifact. However, it is not always perfect in clinical practice. Deep learning-based motion correction is an attractive solution. However, it requires pairs of images with and without motion artifacts, which is difficult in body MRI. Here, we propose a deep learning-based method to remove motion artifact using a simulation of artifacts based on a simple model for respiratory gating failure. Preliminary results showed that the proposed method can remove motion artifacts in respiratory-triggered FSE-T2WI of the liver, which were corrupted by irregular breathing.
Introduction
Respiratory motion has been one of the most challenging problems in abdominal imaging.1 In T2-weighted fast spin echo (T2W-FSE) sequence, an acquisition with single excitation suffers from T2-blur and thus multiple acquisitions at different time periods are needed. However, an inconsistency of respiratory states between data acquisitions results in motion artifacts such as ghosting and blurring. To avoid the problems, respiratory-triggering is performed using respiratory bellows or navigator echoes.2 Unfortunately, even with these techniques, abdominal images can be corrupted by motion in clinical situations especially for patients who have irregular breathing cycle. Recently, deep learning (DL)-based post-processing has attracted much attention in the field of MRI because of its powerful performance and fast computation.3 Retrospective filtering using DL can salvage abdominal MR images corrupted by respiratory motions.4 However, training of neural networks typically requires pairs of motion-free and motion-affected images at the same plane position, which is difficult to obtain in daily clinical practice. Therefore, training sets often consist of a small number of images, limiting the performance of DL-based methods. Here, we propose a combined framework of deep convolutional neural networks and model-based artifact simulation, hereinafter called Motion Artifact Reduction with Convolutional neural network (MARC). In this approach, images with motion artifacts were synthesized in the k-space. Therefore, only motion artifacts-free images are necessary as data source, which mitigate the difficulty of data collection. The aim of this study is to show the feasibility of our method for motion reduction of respiratory-triggered T2W-FSE images of the liver.Methods
This study was approved by the institutional review board. Training data sets were acquired from seven healthy volunteers on a 3 Tesla MRI scanner (SIGNA Premier, GE Healthcare, Chicago, IL, USA) using anterior and posterior array coils. MR data acquisition was performed using a two-dimensional T2W-FSE sequence with bellows-triggering. Each image was reconstructed with 6 shots (6 echo trains) using the following parameters; phase encoding steps, 192; parallel imaging factor, 2; echo train length, 15. The volunteers were told to breathe normally to obtain clean images without motion artifacts. Two of the seven volunteers underwent additional scans, in which they were instructed to breathe irregularly to get corrupted images with respiratory motion artifacts.Our simulation of motion artifacts is based on an anatomical nature of respiratory motion. The dominant component of respiratory motion is translation in the head-foot (HF) direction.5 Therefore, the failure of respiratory triggering should result in the shift of the acquired slices in HF direction. Thus, it is reasonable to synthetically produce an artifact image by replacing an echo train in a slice with that in another slice as shown in Fig. 1. To synthesize k-space with motion artifacts, the k-space data of the clean images was replaced among slices on an echo train by echo train basis with following settings; the probability of the echo train replacement, 0.2; the maximal translation width, 24 mm. The synthesis of k-space data was performed 20 times for each clean image. The synthesized k-space datasets were reconstructed using auto-calibrating reconstruction.6
Network training and validation was done using pairs of motion-free and synthesized motion-affected images. Network testing was based on real artifact images. We use the denoising convolutional neural network7 combined with the aliasing layer8 to handle ghost artifacts.
Results
Fig. 2 shows the learning curves. Both the training and validation loss showed monotone decrease during training and good convergence. The averaged peak signal-to-noise ratio (PSNR) of the images after MARC (DL filtering for artifact reduction) was 36.99 dB (validation sets), while that before MARC was 35.25 dB. The averaged structural similarity (SSIM) index of the images after and before MARC were 0.938 and 0.909, respectively. Fig. 3 shows the feasibility of MARC on test image sets, that were corrupted by real motion artifact during the image acquisition. The proposed method (MARC) successfully filters out motion artifacts, while keeping anatomical structures.Discussion
We proposed a combined framework of deep learning and artifact simulation. In general, typical approaches based on the actual collection of pairs of images with and without motion artifacts require image registration as an additional preparation step before network learning, however, our method requires only motion-free images as data source and can overcome the difficulty of collecting pairs of images with and without artifacts in the conventional approaches.The proposed method in this study assumes translation in the HF direction to be the source of motion artifacts, but it can be extended to take account of another type of parallel motion like abdominal wall movement in anterior-posterior direction.9 The combination of various types of respiratory motion can enhance the capability of simulating motion artifacts and the performance of motion correction.
An extensive clinical evaluation on a larger dataset is required for future works.
Conclusion
The proposed framework combining DL and artifact simulation is a promising approach to develop DL-based motion correction of abdominal images thanks to the ease of data collection.Acknowledgements
No acknowledgement found.References
- McClelland JR, Hawkes DJ, Schaeffter T, King AP. Respiratory motion models: a review. Med Image Anal. 2013;17(1):19-42.
- Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173(1):255-263.
- Kidoh M, Shinoda K, Kitajima M, et al. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn Reson Med Sci. 2019 [Epub ahead of print]
- Küstner T, Armanious K, Yang J, et al. Retrospective correction of motion-affected MR images using deep learning frameworks. Magn Reson Med. 2019;82(4):1527-1540.
- Clifford MA, Banovac F, Levy E, Cleary K. Assessment of hepatic motion secondary to respiration for computer assisted interventions. Comput Aided Surg. 2002;7(5):291-299.
- Beatty PJ, Brau AC, Chang S, et al. A Method for Autocalibrating 2-D Accelerated Volumetric Parallel Imaging with Clinically Practical Reconstruction Times. In Proceedings of the 15th Annual meeting of the ISMRM, Berlin, Germany, 2007;1749.
- Zhang K, Zuo W, Chen Y, Meng D, Zhang L. Beyond a Gaussian Denoiser: Residual Learning of Deep CNN for Image Denoising. IEEE Trans Image Process. 2017;26(7):3142-3155.
- Takeshima H. Convolutional Neural Networks with Aliasing Layers for Correcting Parallel Imaging and EPI Ghost Artifacts. In Proceedings of the 27th Annual meeting of the ISMRM, Montreal, Canada, 2019;4695.
- Tamada D, Kromrey ML, Ichikawa S, Onishi H, Motosugi U. Motion Artifact Reduction Using a Convolutional Neural Network for Dynamic Contrast Enhanced MR Imaging of the Liver. Magn Reson Med Sci. 2019 [Epub ahead of print]
Figures
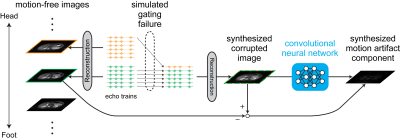
Schemas of Motion Artifact Reduction using a Convolutional neural network (MARC) for respiratory-triggered T2WI: the proposed framework combining deep learning and model-based artifact simulation. Failure of respiratory gating is simulated by replacing an echo train in a slice with that in another slice. This process is based on the fact that respiratory motion is mainly translation in the head-foot direction. Network training can be done by using only motion-free images as data source.

Training and validation loss plotted against the number of epochs. Both losses show monotone decrease during training and good convergence.

Results of motion artifact reduction (MARC) in the images with real artifact by irregular breathing. Image blurring and phase-error-related ghosting artifacts are reduced after applying MARC algorithm. (A) Before MARC, (B) after MARC. (C), (D) Magnified images of the solid boxes in (A), (B), respectively. A yellow arrow indicates the phase-error-related ghosting artifacts.