2592
Prediction of contrast-enhanced liver MRI with CycleGAN1Philips Healthcare, Suzhou, China
Synopsis
Contrast-enhanced MR scans have been commonly used in the diagnosis of liver lesions. However, it has limited use to the patients allergic to the contrast agent. Recently, there are increasing concerns about the safety of the use of Gadolinium-based contrast agents. In this study, we employed a cycle generative adversarial network strategy to test the feasibility of predicting the contrast enhancement images in liver MRI.
Introduction
Contrast-enhanced MR scans have been commonly used in the diagnosis of liver lesions, while the acquisition needs to be carried out in a time window right after the contrast agent injection. It has limited use to the patients allergic to the contrast agent. In addition, there have been more concerns about the safety of the use of Gadolinium-based contrast agents. Previous studies have reported demonstration of post-contrast T1w image generation on brain MR images 1,2. In this study, we aim at predicting the contrast enhanced liver MRI from T2 weighted image with a CycleGAN strategy.Methods
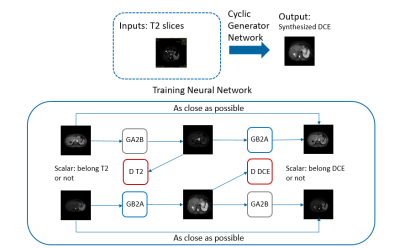
Clinical routine T2-weighted and DCE imaging were performed with 61 subjects on a 3T Ingenia system (Philips Healthcare, the Netherlands). For each subject, the delayed phase of the DCE-MR image (called C-delayed thereafter) was co-registered to the corresponding T2w image using rigid-body registration.A deep generative adversarial network (GAN) was trained to generate a synthesized contrast-enhanced MR image based on T2w image contrast information. A total of 792 T2w and C-delayed image slice pairs were used, with 562 as training set, and 230 as testing set. As shown in Figure 1, we designed the network based on standard cycle generative adversarial network architecture 3 with two generators and two discriminators. We employed the Markovian discriminator (Patch GAN), which yielded more detailed outputs with refined texture. The discriminator consisted of 4 down-sampling layers and a 2D-convolutional layer with kernel size of 3. For loss function, we applied cyclic loss to both generators, and L2 content loss was also applied to prevent generating images not belonging to C-delayed image domain. The loss function was defined as L = λ1L2 + λ2Lcyclic , with constants λ1 = 0.12 and λ2 = 0.1.
To evaluate the similarity of the generated image with the ground truth, both the peak signal-to-noise (PSNR) and the structural similarity index (SSIM) were calculated.
Results
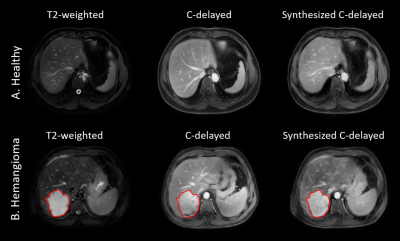
The synthetic C-delayed images had PSNR of 24.3 ± 3.9 and SSIM of 0.860 ± 0.042 compared to the acquired C-delayed images, which was comparable to the reported result in 4. In addition, Figure 2 demonstrated that the trained network predicted the contrast-enhanced images with different cases. For the prediction of a healthy subject case (Fig.2A), the tissue contrast of the predicted image was similar to that of the acquired C-delayed image. For the case of lesions (Fig.2B), the network could predict the post-contrast response in the synthesized C-delayed image with hemangioma lesion.Discussion and Conclusion
In this study, we pioneered the use of a cycle generative adversarial network strategy to generate the contrast enhanced images from T2-weighted images on paired liver MR images. The two metrics PSNR and SSIM measured the contrast enhancement with regard to the ground truth images. In addition, the network could pick up the contrast response behaviors of lesions as shown in case Fig.2B. Some limitations of this method include the smoothness of the synthesized images compared to the original ones, the loss of small vessel structures in the prediction, etc. Future work will focus on including multi-parametric MR information as input to improve the contrast enhancement prediction.Acknowledgements
No acknowledgement found.References
1. Christen, T., Gong, E., Guo, J., Moseley M., Zaharchuk G. Predicting Contrast Agent Enhancement with Deep Convolution Networks. ISMRM 2018 Proceedings.
2. Liu, J., et al., Contrast-free MRI Contrast Enhancement with Deep Attention Generative Adversarial Network. ISMRM 2019 Proceedings.
3. Zhu, J.Y., Park, T., Isola, P., Efros, A.A. Unpaired Image-to-Image Translation using Cycle-Consistent Adversarial Networks. arXiv preprint arXiv:1611.07004; 2017.
4. Salman UI., et al. Image Synthesis in Multi-Contrast MRI with Conditional Generative Adversarial Networks. arXiv preprint arXiv: 1802.01221; 2018.
Figures