2582
Imaging of the intestinal lymphatics following oral consumption of lipids using MRI.1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2School of Pharmacy, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham Biomedical Research Centre, Nottingham University Hospital and University of Nottingham, Nottingham, United Kingdom, 4Nottingham Digestive Disease Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom
Synopsis
It has been shown previously that lipophilic (lipid soluble) drugs administered in lipid-based formulations (or using lipophilic prodrugs) can be delivered in very high concentrations to the intestinal lymphatics. In order for the intestinal lymphatic targeting to be clinically relevant, the response of intestinal lymph following oral administration of lipids needs to be investigated in humans. This is the first study we are aware of using MRI to study changes in lymph nodes following the consumption of a fatty meal. With optimisation this method could provide a novel marker of which nodes could be target for treatment using lipophilic drugs.
Introduction
The lymphatic system plays an important role in the pathophysiology of multiple diseases including lymphomas, cancer metastasis, autoimmune disease and infectious disease. Dietary lipids are preferentially absorbed into the intestinal lymphatics through association with chylomicrons. It has been shown previously that lipophilic (lipid soluble) drugs administered in lipid-based formulations (or using lipophilic prodrugs) can be delivered in very high concentrations to the intestinal lymphatics, where more than 50% of the body’s lymphocytes are harboured1,2. However, it remains unclear precisely which lymph nodes, beyond the superior mesenteric lymph nodes, can be targeted using this approach. In order for the intestinal lymphatic targeting to be clinically relevant, the response of intestinal lymph nodes following oral administration of lipids needs to be investigated in humans. Here we will test the feasibility of identifying any changes in the lymph nodes in response to ingestion of fat and estimate the time course of those changes to inform future studies. The ultimate aim is to identify which lymph nodes could be targeted by lipophilic drug delivery.Aim: to observe changes in the size, number and Apparent Diffusion Coefficient (ADC) of abdominal lymph nodes with time in a pilot study of healthy volunteers after consuming a high-fat meal.
Methods
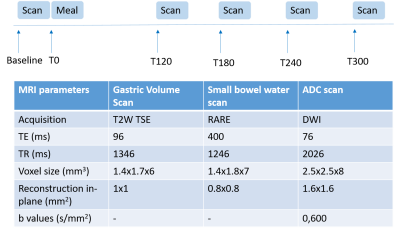
Healthy participants (N=3) were scanned on a Phillips 3T Ingenia (Best, the Netherlands). Images were acquired at baseline and then 120, 180, 240 and 300 minutes after the consumption of a high fat content meal. The meal consisted of 300g creamed rice pudding uniformly mixed with 25g seedless raspberry jam and 30g double cream, and a drink of 100ml orange juice with 240mL of water (Total energy content 518.8kcal, fat content 18.5g, carbohydrate content 76.5g, gastric emptying time of around 2 hours, known from in-house data). To reduce through plane motion, slices were orientated sagittally and respiratory triggering was used. The DWIBS sequence was used to highlight the lymph nodes within the abdomen (diffusion weighted with pre inversion, TI=260ms, for background suppression). Two diffusion weightings (b=0,600 s/mm2) were used to measure ADC across 8 slices (see Figure 1). Gastric emptying and small bowel water3 were also measured to monitor the progression of fat through the GI system. For each lymph node an ROI was drawn on the two diffusion weighted images and the signals used to calculate ADC using the formula $$$ADC = -1/b_{600} ln(S(b_{600})/S(b_0))$$$. The lengths of the major and minor axis of the lymph nodes were also recorded, prior to this a threshold was applied to the image for better definition of the edges of the nodes. All ROIs were drawn in MIPAV4. Differences in the ADC, major and minor axis over time were compared using a one way ANOVA and were corrected for multiple comparisons of the postprandial time points vs baseline if a significant ANOVA was calculated.Results
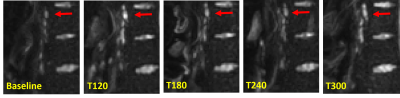
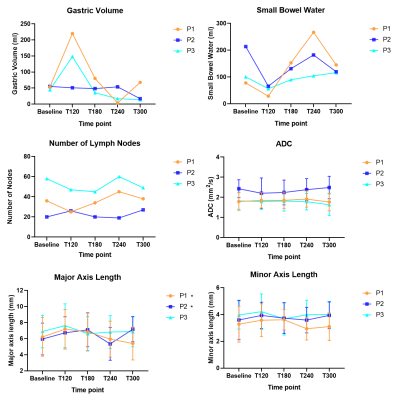
Figure 2 shows lymph nodes identified on DWI images in a healthy participant at baseline and after ingestion of the high fat content meal. On average 37±14 nodes were identified per person at each time point, however the number of nodes seen did vary at different time points, possibly due to increased motion of the nodes due to increased peristalsis after feeding. Figure 3 shows the gastric volume, small bowel water, number of nodes observed, ADC, major axis and minor axis lengths of nodes observed for each participant at each time point. Gastric volume and small bowel water data show the majority of the meal had emptied from the stomach after 2-3 hours (gastric volumes returned to baseline) and the lipids were in the small intestine undergoing digestion (increase in small bowel water content following initial decrease). ADC and minor axis length showed no change over time. The major axis length showed a significant increase between T=0 and T=120 min averaging over all nodes (p=0.03) and a trend for changes with time for participants 1 and 2 (p=0.03 and p=0.02 respectively ANOVA, prior to correction).Discussion
Figure 2 shows nodes near the spine which were relatively fixed. Closer to the bowel the position of the nodes varied more during the experiment, making it difficult to perform a node by node analysis. This data gives a guide to the times at which changes in nodes can be observed and this will inform future studies. However using time points adjusted to personalized gastric emptying could improve sensitivity. Three limitations were present in the study, firstly the number of subjects, secondly the time gap between acquisitions, which could have allowed for the peak enlargement of nodes to be missed and finally only 8 imaging slices were acquired for the diffusion imaging due to time constraints.Conclusion
This is the first study we are aware of using MRI to study changes in lymph nodes following the consumption of a fatty meal. With optimisation this method could provide a novel marker of which nodes could be target for treatment using lipophilic drugs.Acknowledgements
We would like to acknowledge AstraZeneca and GlaxoSmithKline for their supervision and EPSRC for their funding (grant code EP/L01646X/1).References
1. Miura, S., Tsuzuki, Y. & Ishii, H. (1998). Modulation of intestinal immune system by dietary fat intake: relevance to Crohn's disease. J Gastroenterol Hepatol 13(12): 1183-90.
2. Lee, J. B., Zgair, A., Malec, J., Kim, T. H., Kim, M. G., Ali, J.,….Gershkovich, P. (2018). Lipophilic activated ester prodrug approach for drug delivery to the intestinal lymphatic system. Journal of controlled release: official journal of the Controlled Release Society, 286, 10–19.
3. Hoad, C.L., et al., Non-invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol, 2007. 52(23): p. 6909-22.
4. M J McAuli_e, F M Lalonde, D McGarry, W Gandler, K Csaky, and B L Trus. Medical Image Processing, Analysis and Visualization in Clinical Research. In Proceedings 14th IEEE Symposium on Computer-Based Medical Systems. CBMS 2001, pages 381{386, MIPAV. IEEE Comput. Soc. ISBN 0-7695-1004-3. doi: 10.1109/CBMS.2001.941749.
Figures