2580
Hyoscine butylbromide for bowel movement reduction in mouse abdominal MRI1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal
Synopsis
Bowel movement is a source of motion artifacts in mouse abdominal MRI. This work aimed to evaluate fasting and hyoscine butilbromide (Buscopan®) for its reduction. Thirteen mice were imaged in a 9.4T MRI scanner with a FLASH sequence for 90 minutes (~19s temporal resolution): 6 were injected with a Buscopan® bolus, 4 with a bolus and constant infusion, and 3 were food deprived. A single bolus of Buscopan® was effective for bowel movement reduction in mice, for up to 1h, whereas other methods were not effective. These data favor the use of Buscopan® for abdominal MRI in the mouse.
Introduction
Abdominal MRI in the in vivo mouse is frequently hindered by physiological movement such as breathing and bowel motility, which cause blurring and ghosting artifacts1. Several techniques are widely used for respiratory movement compensation, with relatively satisfactory results2,3. However, despite the importance of suppressing bowel movement, there is little consensus on how to achieve it4,5. Fasting and hyoscine butylbromide (Buscopan®) with variable dosages have been proposed, but their efficacy has not been thoroughly assessed6,7. Furthermore, the motion reduction window with Buscopan® is reportedly short (~15minutes). Clearly, suppressing bowel motion over longer periods can be imperative for high quality abdominal imaging. The aim of this study was to develop a consistent protocol for bowel-motion reduction in the mouse and evaluate its performance.Methods
All experiments were preapproved by the institutional and national authorities and in accordance to the European Directive 2010/63.Animal preparation: C57BL/6 mice (N=13, male) weighing 28.4±1.5g and aged ~14weeks, were induced with 5% isoflurane anesthesia mixed with oxygen-enriched (28%) air. Isoflurane maintenance dose was 2-3% and the animals were kept warm with a heating pad. A catheter was inserted intraperitoneally in N=10 animals for Buscopan® administration. Rectal temperature and respiration rate were monitored and kept stable throughout the experiment.
MRI protocol: Animals were imaged in a 9.4T BioSpec scanner (Bruker, Karlsruhe, Germany) with a 40mm-ID linear transmit-receive volume coil (Bruker, Karlsruhe, Germany). Two 1-mm thick coronal slices were positioned in the abdomen and a FLASH sequence (TR/TE=19/2ms, flip angle=20°, FOV=25x25mm2, resolution=120x120µm2, respiratory triggering) was run for 284 repetitions (total duration of 90minutes; temporal resolution of ~19s).
Buscopan® and food deprivation protocols: N=3 mice were food deprived for 5-6h prior to the experiment. The remaining N=10 were injected with Buscopan® (Boehringer Ingelheim, Barcelona, Spain) after 10 min of acquisition: N=6 with a bolus of 0.5mg/kg6, and N=4 with a bolus of 0.17mg/kg immediately followed by a constant infusion of 0.25mg/kg/h until the end of the scan (total dosage of 0.5mg/kg). In the end, all mice recovered from sedation within a few minutes.
Data analysis: Two readers reviewed the acquired images, blinded to the interventions performed on the animals, and defined in consensus the time intervals for presence of significant bowel movement reduction. The time from the first injection of Buscopan® to the start of bowel movement reduction and the duration of effects were then obtained. Kruskal-Wallis testing in SPSS (SPSS Inc., NY, USA) was used to evaluate differences between conditions on duration of effect.
Datasets were also analyzed in MATLAB™ (MathWorks Inc., Natick, MA). Slice images were global signal regressed and low-pass filtered at 0.013Hz to remove occasional high-frequency oscillations caused by breathing. The sum of absolute differences between consecutive images was computed for 10-11min intervals throughout the acquisition and mapped voxelwise for identification of moving regions. Concurrently, the calculated absolute differences were thresholded for identification of significantly moving pixels at each time instant and summed inside those pixels for each animal.
Results
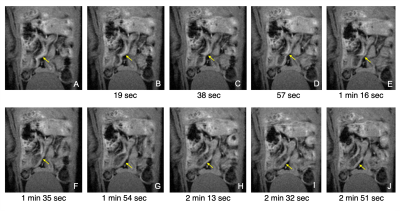
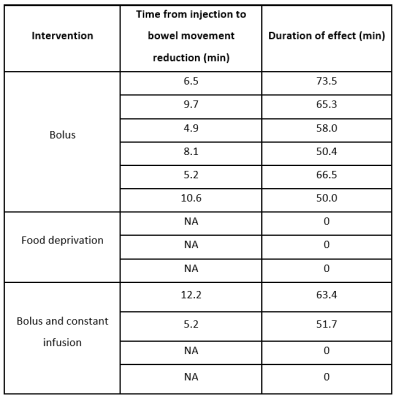
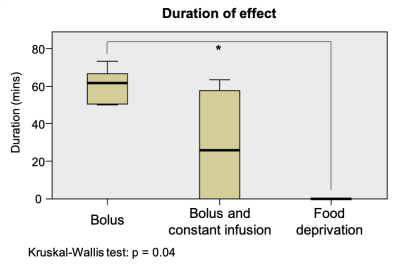
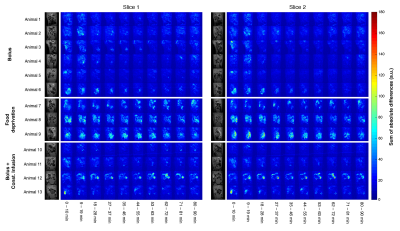
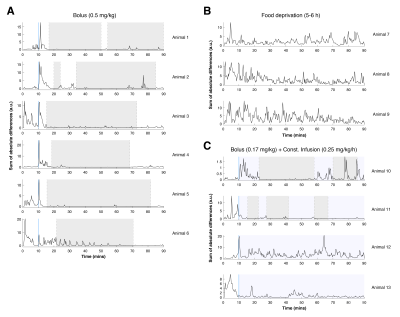
Fig.1 shows raw data for a time period with peristaltic bowel movement considered by the readers in the definition of motion time intervals (Table 1). Kruskal-Wallis testing revealed significant differences in duration of effect between groups (Fig.2).To quantify the effects of each protocol on bowel motility, individual motion maps and time-courses were calculated. Fig.3 and Fig.4 show sustained bowel movement reduction in the bolus group; reduced but irregularly maintained bowel movements in the bolus and constant infusion group; and no noticeable bowel movement reduction in the food deprived group. Half of the mice from the bolus and constant infusion group never showed significant bowel movement reduction (Fig.3(bottom)). Notably, a change in bowel position and increase of bowel motility in the first five minutes after injection was consistent across all animals in the bolus group (Fig.4A). This was not evident in the bolus and constant infusion group (Fig.4C).
Discussion
Our study shows that Buscopan® is effective for bowel movement reduction in the mouse for a duration of >50min, best achieved when administering a single bolus of 0.5mg/kg, taking into account the time interval from injection to start of bowel movement reduction (~7.5minutes) and its duration (~60.6minutes). The smaller bolus dosage injected in the bolus and constant infusion group was possibly not enough to significantly reduce bowel motility. Fasting, suggested by others6 to have comparable effects to Buscopan®, was not effective at all. The differences likely result from our motion quantification strategy involving both subjective and objective criteria. Buscopan® works as a blocker of muscarinic receptors for acetylcholine on smooth muscle cells in the gastrointestinal tract, reducing motility. This effect is evidently more intense and reliable than the motility reduction obtained with fasting.If longer periods of reduced bowel motility are required, repeated bolus injections might not be adequate due to the initial injection effects on bowel movement seen in the bolus group (Fig.4A). Other combinations of bolus and constant infusion dosages should therefore be considered for further testing.
Conclusion
Buscopan® administered in a single bolus is effective for bowel movement reduction in the mouse for up to 1h. These results are promising for motion-sensitive high-resolution abdominal MRI requiring good anatomical definition.Acknowledgements
Funding Support: Champalimaud Foundation; H2020-MSCA-IF-2018, ref:844776.References
1. Wood ML, Henkelman RM. MR image artifacts from periodic motion. Med Phys. 1985;12(2):143-151. doi:10.1118/1.595782
2. Cassidy PJ, Schneider JE, Grieve SM, Lygate C, Neubauer S, Clarke K. Assessment of Motion Gating Strategies for Mouse Magnetic Resonance at High Magnetic Fields. J Magn Reson Imaging. 2004;19(2):229-237. doi:10.1002/jmri.10454
3. Kinchesh P, Allen PD, Gilchrist S, et al. Reduced respiratory motion artefact in constant TR multi-slice MRI of the mouse. Magn Reson Imaging. 2019;60:1-6. doi:10.1016/j.mri.2019.03.018
4. Dear JW, Kobayashi H, Jo S-K, et al. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005;67(6):2159-2167. doi:10.1111/j.1523-1755.2005.00321.x
5. Ellmann S, Langer V, Britzen-Laurent N, et al. Application of machine learning algorithms for multiparametric MRI-based evaluation of murine colitis. PLoS One. 2018;13(10):e0206576. doi:10.1371/journal.pone.0206576
6. Grimm J, Potthast A, Wunder A, Moore A. Magnetic resonance imaging of the pancreas and pancreatic tumors in a mouse orthotopic model of human cancer. Int J Cancer. 2003;106(5):806-811. doi:10.1002/ijc.11281
7. Partecke IL, Kaeding A, Sendler M, et al. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer. 2011;11. doi:10.1186/1471-2407-11-40
Figures