2574
Single-shot T2-weighted Fetal MRI with variable flip angles, full k-space sampling, and nonlinear inversion: towards improved SAR and sharpness1Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 5Harvard-MIT Health Sciences and Technology, Cambridge, MA, United States, 6Institute for Medical Engineering and Science, Cambridge, MA, United States
Synopsis
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE) for rapid T2-weighted imaging to mitigate motion. However, T2-decay and partial fourier induce blurring, while refocusing trains extend total acquisition time due to specific absorption rate (SAR) constraints. Variable flip angles (VFA) with full Fourier acquisitions have been shown to improve image quality, maintain contrast, and accelerate acquisition through SAR reduction. We combine nonlinear reconstruction, VFA, and full Fourier undersampling to improve sharpness and reduce SAR while maintaining similar contrast to HASTE. Simulation and phantom experiments illustrate improved sharpness. Preliminary, in-vivo fetal results demonstrate improved sharpness and SAR with slight tradeoffs in contrast and SNR.
Introduction
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE, SSFSE) for rapid T2-weighted imaging to mitigate fetal motion1. However, T2 decay and partial fourier induce blurring, while the constant refocusing train extends total scan time due the delay between the 20 - 50 sequentially excited slices required to meet specific absorption rate (SAR) constraints.Previous work demonstrated that variable flip angles (VFA) with full Fourier acquisitions and advanced reconstruction techniques improve HASTE image quality, maintain clinically relevant contrast, and accelerate acquisition times through SAR reduction2-5.
We combine nonlinear reconstruction6 with VFA and R = 4/R=3 uniform, full Fourier under-sampling to analyze if image sharpness and SAR can be improved while maintaining similar contrast to HASTE in fetal MRI. Simulation and phantom results illustrate improved sharpness and qualitatively similar contrast. Preliminary in-vivo fetal experiments demonstrate potential for improved sharpness and lower SAR with slight tradeoffs in contrast and lower SNR in comparison to HASTE.
Methods
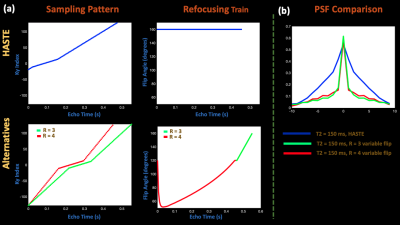
Sequences to TestFigure 1 (a) depicts the alternative sequences we compared to HASTE. HASTE acquires 86 ky lines with 9/16 partial fourier, TE = 85 ms, 24 auto-calibration lines, R = 2, and constant 160 degree refocusing pulses. On the other hand, the R = 4 sequence samples 82 ky lines, including 24 auto-calibration lines and no partial Fourier. The R = 3 sequence does the same with 100 ky lines. The alternative sequences also incorporate VFA, structured from literature2,3,7. All sequences had an echo spacing of 5.56 ms. The R = 3 and R = 4 sequences respectively achieve 2.8 and 3.8 times decrease in relative SAR, computed as the sum of squares of the refocusing angles divided by echo train length2. Finally, we compared the relative contrast difference8 at the echo when the center of k-space is sampled in EPG9 simulated signal evolution for T2 values of 1 s and .2 s, representative of fetal CSF and brain10. HASTE achieves relative contrast of .37, while the VFA sequences achieve approximately .42.
Nonlinear Inversion
Regularized nonlinear inversion (NLINV) resolves the R = 3/R = 4 undersampling in the alternative sequences6. NLINV has been shown to improve upon GRAPPA8 and SENSE9 by solving $$$y = F(x)$$$ (with $$$x = $$$ $$$[p, $$$ $$$c_1 ... c_N]$$$) to jointly estimate estimate the image ($$$p$$$) and coil sensitivities ($$$c_i$$$), where $$$y$$$ is the measured data, and $$$F$$$ maps $$$x$$$ to $$$y$$$. Since $$$F$$$ is a nonlinear operator, the problem is solved using a regularized Gauss-Newton method with a Sobolev norm applied to the coil sensitivity estimates6.
Experiments
All experiments acquired data with the standard HASTE sequence, the alternative R = 3/R = 4 sequence with VFA, and the R = 4 sequence with constant 160 degree flip angles. Numerical data was simulated from adult brain realistic T1, T2, proton density, and sensitivity maps with 256 x 256 mm FOV, 1 x 1 mm resolution, and 256 x 256 matrix size9,11.
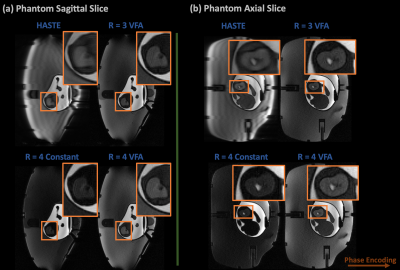
A pregnant abdomen phantom was scanned with 300 x 300 mm FOV, 1.17 x 1.17 x 3 mm resolution, and 256 x 256 matrix size in the scanner sagittal and axial orientations.
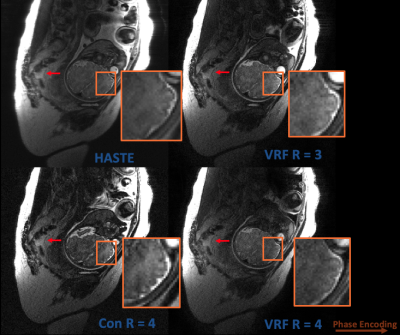
With IRB approval, a woman 38 weeks into gestation was scanned with 665.6 x 332.8 mm FOV, 1.3 mm in-plane resolution, 512 x 256 matrix size, and slice thicknesses of 3 mm and 5 mm.
For phantom and in-vivo experiments, a 3T Siemens Skyra scanner was used. The alternative techniques were reconstructed with NLINV, and HASTE was reconstructed with GRAPPA and POCS partial Fourier.
Results
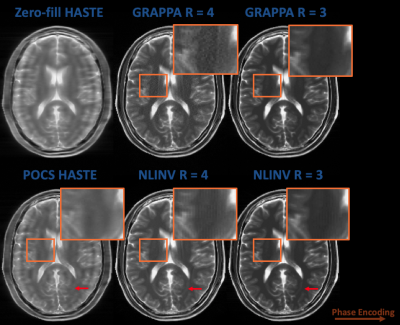
Figure 2 compares the alternative R = 3/R=4 technique reconstructed using GRAPPA and NLINV with zero-filled and POCS HASTE in simulation. The alternative techniques reduce image blurring and maintain reasonable contrast, while NLINV reduces noise amplification at R = 4, in comparison to GRAPPA.Figure 3 shows HASTE, R = 3/R = 4 with VFA, and R = 4 with constant 160 degree flip angles at both sagittal and axial orientations in an abdomen phantom. Again, the alternative sampling scheme with VFA and no partial fourier improves image quality and maintains reasonable contrast.
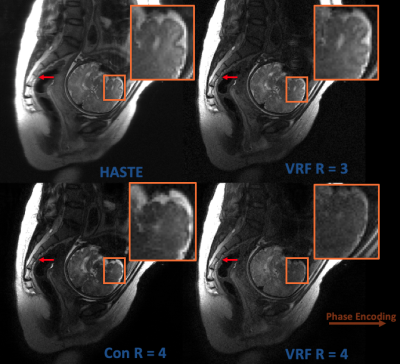
In Figure 4, HASTE, R = 3/R = 4 with VFA, and R = 4 with constant flip angles are compared in a 3 mm slice thickness, fetal acquisition. Image sharpness is improved and some level of contrast is maintained, but HASTE qualitatively exhibits higher SNR. Figure 5 performs the same comparisons at 5 mm slice thickness; the alternative techniques maintain the aforementioned benefits with qualitatively less SNR penalty.
Discussion
Full Fourier, uniformly under-sampled R = 3/R = 4, VFA acquisitions reconstructed with nonlinear inversion was compared to HASTE. Simulation and phantom results demonstrated potential for the technique to improve image sharpness, and in-vivo experiments demonstrated improvements in sharpness and reduced SAR, but with tradeoffs in contrast and SNR. The R=3/R=4 with VFA acquisitions are also expected to have shorter total scan times, due to 2.8x and 3.8x relative SAR reduction which will shorten the delay between the 20-50 excited slices2. Future work will involve precise quantification of scan time reduction from decreased SAR and analysis with Radiologists about the clinical impact of the technique’s contrast and SNR tradeoffs.Acknowledgements
This work was supported in part by research grants NIH R01 EB017337, U01 HD087211, R01HD100009, the NVidia Corporation for computing support, and the Skolkovo Institute of Science and Technology Next Generation Program. In addition, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1122374. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science FoundationReferences
1. Patel MR, Klufas RA, Alberico RA, Edelman RR. Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: comparison with fast spin-echo MR in diseases of the brain. AJNR Am J Neuroradiol. 1997;18: 1635–1640.
2. Loening AM, Saranathan M, Ruangwattanapaisarn N, Litwiller DV, Shimakawa A, Vasanawala SS. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J Magn Reson Imaging. 2015;42: 1747–1758.
3. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55: 1030–1037.
4. Chen F, Cheng JY, Taviani V, Sheth VR, Brunsing RL, Pauly JM, et al. Data‐driven self‐calibration and reconstruction for non‐cartesian wave‐encoded single‐shot fast spin echo using deep learning. J Magn Reson Imaging. 2019;6: 698.
5. Chen F, Taviani V, Tamir JI, Cheng JY, Zhang T, Song Q, et al. Self-Calibrating Wave-Encoded Variable-Density Single-Shot Fast Spin Echo Imaging. Journal of Magnetic Resonance Imaging. 2018. pp. 954–966. doi:10.1002/jmri.25853
6. Uecker M, Hohage T, Block KT, Frahm J. Image reconstruction by regularized nonlinear inversion—joint estimation of coil sensitivities and image content. Magn Reson Med. 2008;60: 674–682.
7. Tamir JI, Uecker M, Chen W, Lai P, Alley MT, Vasanawala SS, et al. T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging. Magn Reson Med. 2017;77: 180–195.
8. Keerthivasan MB, Saranathan M, Johnson K, Fu Z, Weinkauf CC, Martin DR, et al. An efficient 3D stack-of-stars turbo spin echo pulse sequence for simultaneous T2-weighted imaging and T2 mapping. Magn Reson Med. 2019;82: 326–341.
9. Weigel M. Extended phase graphs: dephasing, RF pulses, and echoes‐pure and simple. J Magn Reson Imaging. 2015. Available: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmri.24619
10. Jeffrey N Stout, Congyu Liao, Esra Abaci Turk, Borjan Gagoski, P. Ellen Grant, Lawrence L. Wald, and Elfar Adalsteinsson. T1 and T2 mapping of the placenta and fetal brain with magnetic resonance fingerprinting (MRF) during maternal hyperoxia. In Proceedings of the 27th Annual Meeting of ISMRM. : 1182.
11. Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17: 463–468.
Figures