2566
Shape and Volume Analysis of the Fetal Brain Pre and Post Fetal Spina Bifida Surgery1Institute for Women's Health and Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom, 2School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, London, United Kingdom, 3Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom, 4Department of Obstetrics and Gynaecology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium, 5Department of Radiology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium
Synopsis
Thorough assessment of the fetal central nervous system is required to select the most suitable candidates for fetal spina bifida surgery, and for post-operative monitoring to predict outcome. Children with myelomeningocele exhibit difficulties in cognitive performance, and motor skills1. This is related to the Chari II malformation and ventriculomegaly2,3. Our aim is to determine if MRI technology can quantify volume, surface area, and shape of cerebral structures before and after fetal spina bifida surgery. We explore if these parameters can be used as potential biomarkers for efficacy of fetal surgery by correlating with herniation level.
Introduction
Myelomeningocele (MMC) is the most frequent form of open spina bifida entailing an extrusion of the spinal cord into a CSF filled sac4,5. Fetuses with MMC have a constellation of brain anomalies comprising the Chiari II malformation and variable degrees of ventriculomegaly2, 3. Chiari II malformation is characterised by a small posterior fossa and hindbrain herniation in which the cerebellum, medulla and fourth ventricle are displaced caudally into the spinal canal with elongation of the pons and fourth ventricle6,7.The downward displacement is believed to contribute to hydrocephalus development by obstructing normal CSF flow1.Neonates and infants with MMC often require treatment of hydrocephalus by ventriculoperitoneal shunts or, at older age, ventriculostomy. Children with MMC exhibit difficulties in cognitive performance, gross, and fine motor skills1due to the above described brain anomalies2,3,8. Hydrocephalus causes mechanical stretching of brain parenchyma leading to damage of white matter tracts. Additionally, diffusion tensor imaging studies9 have shown abnormal white matter development in MMC fetuses. Mechanical compression of the cerebellum due to herniation may also lead to ischemic changes10causing cognitive and motor deficits.The Management of Myelomeningocele Study, MOMS, provided level I evidence that fetal surgical closure of the defect in selected fetuses, as opposed to after birth, reduces ventriculoperitoneal shunt requirement by 40% and presence of hindbrain herniation at 12 months6,7.With an increasing number of prenatal operations being performed, though not all benefitting the fetus, it may be useful to better understand the evolution of the fetal brain after surgery using ultrasound and MRI. This may eventually improve case selection due increased knowledge of the pathophysiology in these fetuses. MRI however has limitations due to fetal motion and limited spatial resolution. Our study aims to combat that challenge and generate volumetric analysis of the white matter, ventricles and cerebellum using novel MRI methods.Methods
Twelve MMC fetuses had an MRI before surgery (Mean: 23+6 ± 1+7 weeks+days, (range 22+1–25+6)),and after surgery (26+1 ± 1+3 weeks, (24+1– 29+4)). These were compared to an early (23+3 ± 1+4 weeks, (21+5-25+3)) and late control group (28+5 ± 1+3 weeks, (26+0-30+2)) without central nervous system abnormalities.T2-weighted single-shot fast spin-echo (SSFSE) was performed of the fetal brain in multiple orientations with at least one stack in axial, coronal and sagittal planes. Slice thickness was 3mm and total acquisition time was thirty-forty minutes. A novel automated super resolution reconstruction (SRR) algorithm11,12was used to build 3D volumes of the fetal brain. Rigid slice-to-volume registration correcting for fetal motion was used to generate an SRR image in standard anatomical orientation. From this SRR volume, we carried out automatic segmentation of the white matter, ventricles and cerebellum, using template brain segmentations13,14.Segmented structures where checked and manually corrected, and meshes were generated15 allowing for calculation of volume, surface area, and surface area/volume ratio13.Shape parameter was defined as surface area/volume.Hindbrain herniation level was measured perpendicularly from the foramen magnum to the lowest cerebellar portion on SRR images in the midsagittal plane16. Paired t-test was performed to compare mean differences between groups. Statistical significance was set at 5%.Correlations between herniation change and shape parameter were assessed using Pearson’s correlation coefficient (r).Results
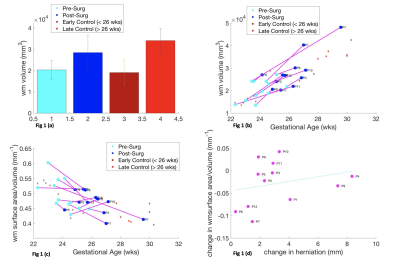
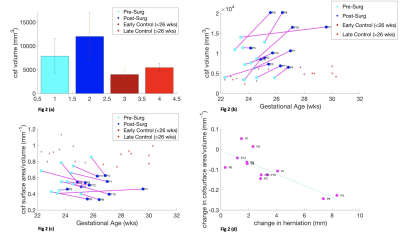
Figure 1 illustrates that white matter MMC volume increases after surgery 95%CI 2.03x104 mm3 to 2.84x104 mm3, at a rate of 0.31x104/week (p = 0.0064) but less than in controls 95%CI 1.91x104 mm3 to 2.41 x104 mm3, at a rate of 0.35x104/week (p=8.085x10-5) (Fig1a/b). MMC white matter exhibit no significant change in shape parameter (p = 0.073),(Fig1c). Cerebellar herniation level and shape are weakly correlated (r= 0.2564, p= 0.4212) (Fig 1d).Figure 2 suggests that MMC ventricular volume increases after surgery 95%CI 0.79x104 mm3 to 1.20x104mm3 (p=0.033), at a rate of (0.18x104 mm3/week), more so than controls 95%CI 0.40 x104 to 0.53 x104 mm3 (p=0.018), at a rate of (0.033x104/week),(Fig2a/b). MMC ventricles after surgery demonstrate a significant change in shape parameter, p=0.0025 (Fig2c). Cerebellar herniation level and shape parameter are negatively correlated (r=-0.7997, p= 0.0018) (Fig2d).
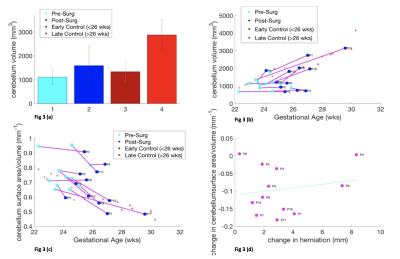
Cerebellar volume in MMC fetuses increases after surgery 95% CI 1.11x103 to 1.59x103 mm3 (p=0.0674), at a rate of 0.21mm3/week, but less than in controls 95%CI 1.34x103 to 2.88x103mm3 (p=2.6x10-5), at a rate of 0.33 mm3/week (Fig 3a/b). MMC cerebella after surgery follow a similar trajectory of shape change to controls, relative to their initial shape parameter, p=5.6x10-4(Fig3c). Cerebellar herniation level and shape are weakly correlated (r=0.1778, p=0.5804) (Fig3d).
Discussion and Conclusion
We have shown that MMC white matter surface area/volume pre and post-surgery changes the least, in comparison to ventricles and cerebella of MMC cases, in relation to controls. Ventricular volume increases significantly after surgery and the surface area/volume is negatively correlated with herniation level. Moreover, change in cerebellar shape is demonstrated after MMC surgery, although it is weakly correlated with hindbrain herniation. Although ventriculomegaly does not reduce once established after surgery, modification of CSF hydrodynamics may decrease ventricular dilatation rate potentially leading a reduction in cerebellar herniation. Additional and longer term longitudinal post-operative antenatal MRI is required to support this theory. Additionally, more data is required to support the use of cerebellar shape parameter as a potential biomarker of surgical efficacy and to investigate the functional significance of anomalous cerebellar development.Acknowledgements
This work is part of the Guided Instrumentation of Fetal Therapy and Surgery (GIFT-Surg) project, funded by the Wellcome Trust [203148/Z/16/Z; 203145Z/16/Z; WT101957] and Engineering and Physical Sciences Research Council (EPSRC) [NS/A000049/1; NS/A000050/1; NS/A000027/1; EP/L016478/1]. Wellcome/EPSRC centre for Interventional and Surgical Sciences (WEISS), and Kings Undergraduate Research Fellowship (KURF) Scheme.
References
1.Wills, K.E., et al., Intelligence and achievement in children with myelomeningocele. J Pediatr Psychol, 1990. 15(2): p. 161-76.
2.Geerdink, N., et al., Essential features of Chiari II malformation in MR imaging: an interobserver reliability study--part 1. Childs Nerv Syst, 2012. 28(7): p. 977-85.
3.Geerdink, N., et al., Interobserver reliability and diagnostic performance of Chiari II malformation measures in MR imaging--part 2. Childs Nerv Syst, 2012. 28(7): p. 987-95.
4.Rethmann, C., et al., Evolution of posterior fossa and brain morphology after in utero repair of open neural tube defects assessed by MRI. Eur Radiol, 2017. 27(11): p. 4571-4580.
5. Zarutskie, A., et al., Prenatal brain imaging for predicting need for postnatal hydrocephalus treatment in fetuses that had neural tube defect repair in utero. Ultrasound Obstet Gynecol, 2019. 53(3): p. 324-334.
6. Adzick, N.S.T., E.A.; Spong, C. Y.; Brock III, J. W.; Burrows, P. K.; Johnson, M. P.; Howell, R. N.; Farrell, J. N.; Dabrowiak, M.E.; Sutton, L.N.; Gupta, N.; Tulipan, N.B.; D’Alton, M.E.; Farmer, D.L., A Randomised Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N Engl J Med, 2011. 364: p. 993-1004.
7. Sutton, L.N.A., N. S.; Bilaniuk L. T.; Johnson, M. P.; Cromblehome, T. M.; Flake, A. W., Improvement in Hinbrain Herniation Demonstrate by Serial Fetal Magnetic Imaging Following Fetal Surgery for Myelomeningocele. JAMA, 1999. 282: p. 1826-1831.
8. Joyeux, L., et al., Fetal surgery for spina bifida aperta. Arch Dis Child Fetal Neonatal Ed, 2018. 103(6): p. F589-F595.
9. Woitek, R., et al., Fetal diffusion tensor quantification of brainstem pathology in Chiari II malformation. Eur Radiol, 2016. 26(5): p. 1274-83.
10. Juranek, J. and M.S. Salman, Anomalous development of brain structure and function in spina bifida myelomeningocele. Developmental Disabilities Research Reviews, 2010. 16(1): p. 23-30.
11. Ebner, M., et al., An Automated Localization, Segmentation and Reconstruction Framework for Fetal Brain MRI, in Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. 2018. p. 313-320.
12. Ebner, M., et al., An automated framework for localization, segmentation and super-resolution reconstruction of fetal brain MRI. NeuroImage, 2019.
13. Orasanu, E., et al., Cortical folding of the preterm brain: a longitudinal analysis of extremely preterm born neonates using spectral matching. Brain Behav, 2016. 6(8): p. e00488.
14. Kuklisova-Murgasova, M., et al., A dynamic 4D probabilistic atlas of the developing brain. Neuroimage, 2011. 54(4): p. 2750-63.
15. Yushkevich, P.A., et al., User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage, 2006. 31(3): p. 1116-28.
16. Aertsen, M., et al., Reliability of MR Imaging-Based Posterior Fossa and Brain Stem Measurements in Open Spinal Dysraphism in the Era of Fetal Surgery. AJNR Am J Neuroradiol, 2019. 40(1): p. 191-198.
Figures