2555
Natural abundance 13C MRS based glycogen detection in Pompe disease: Test/retest characterization in a Gaa KO mouse model
Deanne Lister1, Prodromos Parasoglou2, Andrew Baik2, Katherine Cygnar2, Aris Economides2, Brendan Cook1, Sven Macholl1, and Patrick McConville1
1Molecular Imaging Center, Invicro, A Konica Minolta Company, La Jolla, CA, United States, 2Regeneron Pharmaceuticals, Tarrytown, NY, United States
1Molecular Imaging Center, Invicro, A Konica Minolta Company, La Jolla, CA, United States, 2Regeneron Pharmaceuticals, Tarrytown, NY, United States
Synopsis
Pompe disease is a debilitating condition that results in abnormally high tissue glycogen concentrations and is the subject of significant therapeutic focus by the pharma industry, including promising gene therapies. Non-invasive biomarkers that are specific and sensitive are sought after to address major gaps in early phase clinical trials. We characterized 13C MRS measurements of muscle glycogen concentration as potential biomarkers in Pompe disease, including test/re-test measurements and correlation with tissue biochemical measurements. Strong specificity and reasonable reproducibility was found, suggesting potential for 13C MRS to be used in Pompe disease patients.
Introduction
Pompe disease, glycogen storage disorder type II, is a rare multisystem disease occurring in approximately 1 in 40,000 births and with symptoms presenting anywhere from infancy to adulthood. Characteristic disease complications include progressive cardiac and respiratory dysfunction, and skeletal muscle weakness. Alterations of the GAA gene that encodes the information for production of the acid alpha-glucosidase results in a shortage of the enzyme which is needed to convert glycogen to glucose in the lysosome. This leads to build-up of glycogen in muscle tissue and ultimately results in progressive tissue dysfunction over time. In this project we built upon recent work (Baligand, et al.) by characterized 13C MR spectroscopy in both wild type and diseased mice (GAA KO mouse) to evaluate its potential for use in Pompe disease clinical trials, where there is a lack of non-invasive and predictive biomarkers.Methods
We sought to measure the disease specificity, dynamic range and correlation with standard measures for several MRS biomarkers in diseased vs wildtype mice. This included test/re-test reproducibility measurements as well as correlation of the 13C MRS biomarkers with biochemical tissue glycogen measurements. 13C MRS was performed on Gaa-/- KO mice (N=5) and wild type mice (N=8) at 33-36 weeks of age using a Bruker Biospec 11.7T MRI system. A 20mm 13C surface coil was used for detection of naturally abundant glycogen in combination with a 1H volume coil for anatomical reference and proton decoupling. The hind limb muscles were imaged by placing the mice laterally directly over the surface coil, ensuring coverage of the quadriceps and gastrocnemius. 13C spectra were acquired for 1h using a proton decoupled single pulse acquisition with TR = 500ms, acquisition bandwidth = 25kHz with 1024 acquisition points. Proton decoupling was performed using an Mlev6 CPD sequence with decoupling duration and power of 41ms and 15W, respectively. Both SNR and peak area (calculated using Mnova 11) were calculated following a Lorentzian deconvolution. A 200mM 1-13C-sodium acetate reference phantom was also included for additional normalization of both SNR and peak area. Repeat imaging was performed 1-3 days after the initial acquisitions to assess the reproducibility of the measurements. Following imaging, mice were euthanized, and the quadriceps and gastrocnemius muscles were harvested and flash frozen for glycogen concentration measurements. Tissues were lysed on a benchtop homogenizer with stainless steel beads in distilled water, and boiled and centrifuged to clear debris. Glycogen measurements were performed fluorometrically using a commercial kit according to manufacturer’s instructions (Biovision K646).Results
13C MRS was successful in detection of glycogen in both knockout and wild type mice with all calculated parameters (including normalization with acetate standard) showing high reproducibility from subject to subject and high test/re-test reproducibility (see Table). Measurement of relatively low glycogen concentrations in wild type mice were challenging due the low SNR. Despite this, a correlation was observed between SNR and biochemical analysis of tissue glycogen concentration (r=0.83).Discussion and Conclusions
We have shown that 13C MRS can be used to detect elevated naturally abundant muscle glycogen levels in mice with glycogen storage disorder type II disease, and that measurements are repeatable with reasonable accuracy. Variability is thought to be driven mostly by small variations in sensitivity from the exact positioning of the RF surface coil over the muscles, and additional variability in the accuracy of the peak area calculations for the lower glycogen concentrations in WT mice. MR spectroscopy measurements correlated with biochemical analysis of glycogen concentration in skeletal muscle in wild type mice. These results suggest 13C MRS for detection of naturally abundant glycogen without any 13C labeling could present viable biomarker options for use in early phase Pompe disease clinical trials both for stratification and as secondary response assessment.Acknowledgements
No acknowledgement found.References
1. Baligand C., Todd A.G., Lee-McMullen B., Vohra R.S., Byrne B.J., Falk D.J., Walter G.A. 13C/31P MRS Metabolic Biomarkers of Disease Progression and Response to AAV Delivery of hGAA in a Mouse Model of Pompe Disease. MOL THER-METH CLIN D, 7(15), 42–49.Figures
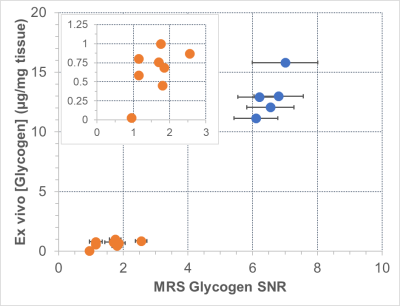
Figure: Correlation between muscle glycogen concentration (measured ex vivo by
biochemical assay) and 13C MRS glycogen peak SNR for wild type (orange circles,
and see inset for more detail) and GAA KO/diseased subjects (blue squares). The
two measurements for glycogen generally correlated, including a statistically
significant correlation in the wild type data. Importantly, the specificity of
the 13C MRS SNR as a biomarker was very high and suggests utility for use in
stratification and response in patients.
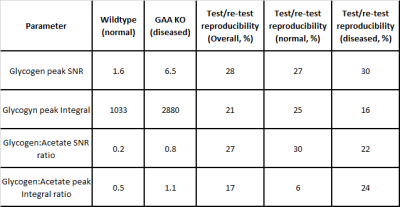
Table Mean
glycogen peak SNR and area, and glycogen:acetate SNR and peak areas ratio for
wildtype and GAA KO (disease model) mice. Mean test/re-test reproducibility is
shown for each parameter.