2542
Is Liver Function Affected by Resective Surgery? – Hepatocyte Uptake and Efflux to Bile in Liver Cancer Determined Using Gadoxetate Enhanced MRI
Christian Simonsson1,2, Markus Karlsson1,3, Bengt Norén1, Gunnar Cedersund2, Per Sandström4, Anna Lindhoff Larsson4, Nils Dahlström1,3, and Peter Lundberg1,3
1Departments of Radiation Physics, Radiology, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden, 2Department of Medical Engineering, Linköping University,, Linköping Univeristy, Linköping, Sweden, 3Center for Medical Image Science and Visualization (CMIV), Linköping Univeristy, Linköping, Sweden, 4Department of Surgery, Department of Clinical and Experimental Medicin, Linköping University, Linköping, Sweden
1Departments of Radiation Physics, Radiology, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden, 2Department of Medical Engineering, Linköping University,, Linköping Univeristy, Linköping, Sweden, 3Center for Medical Image Science and Visualization (CMIV), Linköping Univeristy, Linköping, Sweden, 4Department of Surgery, Department of Clinical and Experimental Medicin, Linköping University, Linköping, Sweden
Synopsis
Just after surgery there is a period were the remnant tissue needs to match the requirement of normal liver function while recovering. This could lead to fatal consequences after too extensive surgical procedures, or due to insufficient liver function. In contrast, it might also be the case that the surgical procedure is too conservative because the predicted liver function is underestimated. This would not have been the case if it was possible to use more precise measures of liver function. We investigated the capabilities of gadoxetate enhanced MRI for determining liver function pre- and post-surgery using two separate approaches.
Introduction
A range of severe liver diseases are discovered only in very late stages, and they are associated with relatively subtle clinical symptoms. At a late stage the only remaining treatment may be resective liver surgery. Just after surgery there is a period were the remnant tissue needs to match the requirement of normal liver function while recovering. This could lead to fatal consequences after too extensive surgical procedures, or due to insufficient liver function.This would not have been the case if it was possible to use more precise measures of liver function. Thus, it would be a major step forward to be able to much more accurately characterize global and regional liver function prior to surgery. To this cause, we investigated the capabilities of determining liver function pre- and post-surgery using two different approaches, whole-body quantitative modeling and a normalized hepatic signal approach.Methods
Patients were aged 42-76 y (both M/F). Preoperative (total) bilirubin was 3-26 µmol/L, and the subjects had a wide range of conditions, including metastasis (HCC, B-cell lymphoma, colon, rectal, and medullary thyroid cancer, etc). Four obtained postoperative chemotherapy, one recurrence, and the 90 days mortality in the cohort was two.Magnetic Resonance was performed on a 3 T Philips Ingenia MR-scanner using a phased-array body coil. Gadoxetate‑enhanced images were acquired using an axial breath-hold, fat-saturated, T1-weighted, 3D gradient echo sequence. Typical image parameters included flip angle: 10°, repetition time: 4.2 ms, echo time: 2.0 ms, SENSE factor: 1.7, field of view: 300x200x350 mm3. DCE-MRI were acquired before and after a bolus injection of gadoxetate at a standard clinical dose (0.025 mmol/kg body weight). The post‑injection images included arterial and portal venous phasesas well as images acquired e.g., at 10 min. ROIs were placed in each of the eight Couinaud segments, plus three ROIs in the spleen, by an experienced radiologist (BN).
Patients (n=16, fasting) with liver cancer were examined a few days before resective surgery using DCE-MRI, following a bolus injection of a hepatocyte specific contrast agent Gd-EOB-DTPA (gadoxetate) pre- and post-surgery (within 3-7 days). However, many patients declined to perform the post-surgical examination mainly because of the physiological and mental load of the treatment procedure as well as the severity of the condition. The local ethics committee approved this study, and written informed consent was obtained from all patients.
We have previously developed methods for quantitatively determine global and regional liver function [1]. Using this methodology, data of gadoxetate uptake in liver and spleen pre- (n=16) and post- (n=5) resective liver surgery was analyzed by estimating parameters in a ‘non-linear mixed effect’ (NLME) mechanistic mathematical model. The model was based on a minimal number of separate compartments and described by ordinary differential equations (Fig 1a). An example of model fit to patient data after parameter estimation is shown in Fig 1b. This methodology allowed for the quantification of influx (kin) and efflux (keff) of gadoxetate for the liver.
We also used alternative phenomenological measures of late hepatobiliary uptake and blood plasma clearance for the comparison. Normalised (S) signal intensity measurements from eight liver and three spleen ROIs were used to calculate the quantitative liver-spleen contrast ratio LSC, see Eq. 1, for 10 or 20 minutes after gadoxetate injection.
Results
The whole body-model we used, and typical uptake curves, are shown in Fig.1; the kin parameter governs the influx rate into the hepatocyte compartment of the model and keff governs the rate efflux of gadoxetate to the bile (Fig. 1). Fig. 2 shows that for the subset of patients with data from both pre- and post-surgery kin decreased and keff increased. In Fig. 3a and 3b the LSC10 and LSC20-ratios for all patients are shown. The first group includes both patients that accepted and declined the post-surgery examination which was offered to all, thus post-surgery examination was performed on a subset. Fig. 3a shows only trend in LSC10 ratio between pre- and post-examination (p=0.085). In contrast, the difference in LSC20 ratio (Fig. 3b), is significant (p= 0.003). Figs. 3c and 3d shows the change in the ratio between pre- and post-surgery examinations.Discussion
Differences in liver function between pre- and post-surgical examinations were observed using both methods: Using the NLME modeling approach, a non-significant trend was observed for the parameters describing global liver function; kin decreased and keff increases. More post-surgery data would be required to strengthen our conclusions.The results obtained using the LSC-ratio approach showed significant liver function differences between pre- and post-examinations, with a lower post-surgical LSC-ratio.This agrees with the changes in rates as it suggests that there is an increased clearance rate of gadoxetate. In the future more multimodal data and clinical information will be added to build a more coherent picture of the challenge an extensive liver surgery will impose on liver function.Conclusion
We have shown that there is a subtle, but significant effect of resective surgery on liver function. If gadoxetate enhanced imaging is accepted as a conventional clinical procedure immediately prior to surgery in the future, we believe that the procedure may provide better clinical outcome as the surgeons will be able to better judge the functional hepatic reserve when planning the procedure.Acknowledgements
No acknowledgement found.References
[1] Forsgren MF, Karlsson M, Dahlqvist Leinhard O, Dahlström N, Noren B, Romu T, Ignatova S, Ekstedt M, Kechagias S, Lundberg P, Cedersund G, Model-inferred mechanisms of liver function from magnetic resonance imaging data: Validation and variation across a clinically relevant cohort. PL oS Comp Biol, June 2019.Figures
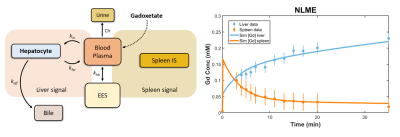
Fig. 1 (Left panel) The whole-body model that was used for NLME-modeling. EES, extra-cellular, extravascular space; kin, influx of gadoxetate; keff, efflux of gadoxetate to bile; khp, reflux from hepatocyte to blood plasma. (Right panel) Data and model describing hepatocyte and spleen signals, respectively.
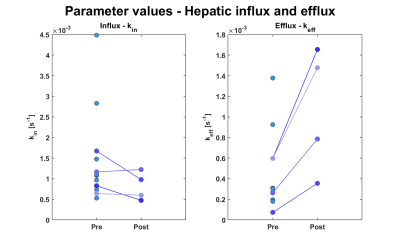
Fig. 2 Hepatocyte influx rate and efflux rate to bile determined using NLME. The measurements were performed within a few days prior to, and after surgical resective procedures. Data were obtained from eight ROIs in respective Couinaud segments.
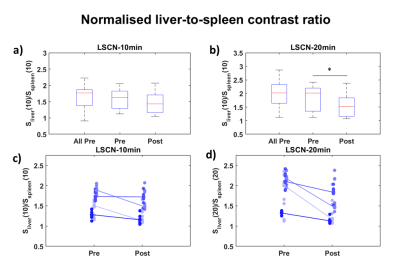
Fig. 3 Normalized signals from eight ROIs in different Couinaud segments and three spleenic ROIs, pre- and post-resective
surgery. Two different time points were used for the analysis (10 and
20 minutes). (a) and (b) shows the different groups, all, pre,
and post-surgery. (c) and (d) shows the corresponding Pre- and post-distributions.
Eq. 1 Quantitative liver-spleen contrast ratio.