2540
Breath-hold MRCP using Accelerated 3D-Spiral Turbo Spin-Echo Imaging1University of Virginia, Charlottesville, VA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Current free-breathing, respiratory-triggered, heavily T2-weighted 3D fast/turbo spin-echo acquisitions work well in many patients for MRCP, but non-diagnostic results are obtained in some patients, particularly those with irregular breathing patterns during the several-minute acquisition. Thus, there has been renewed interest in 3D MRCP techniques that can be completed within a single breath-hold period as an alternative for patients in whom free-breathing techniques are inadequate. This work demonstrates that breath-hold 3D MRCP based on a 3D stack-of-spirals turbo spin-echo acquisition, which uses in-plane acceleration to achieve a reasonable breath-hold time, can provide good image quality for evaluation of major ductal structures.
Introduction & Purpose:
Magnetic resonance cholangiopancreatography (MRCP) is routinely used for clinical assessment of the biliary and pancreatic duct systems. Current approaches often use a free-breathing, heavily T2-weighted 3D fast/turbo spin-echo acquisition that is respiratory triggered. While this type of acquisition works well in many patients, non-diagnostic results are obtained in some patients, particularly those with irregular breathing patterns during the several-minute acquisition time. Recently, there has been renewed interest in 3D MRCP techniques that can be completed within a single breath-hold period1,2 as an alternative for patients in whom current free-breathing techniques are inadequate. The purpose of this work was to: (1) explore the feasibility of breath-hold 3D MRCP based on a 3D stack-of-spirals turbo spin-echo acquisition that uses in-plane acceleration to achieve a reasonable breath-hold time; and (2) investigate the influence of encoding order on artifacts from fluids, such as in the stomach or intestines, that move during the breath-hold period in some subjects.Methods:
The prototype 3D stack-of-spirals turbo spin-echo acquisition was based on a commercial version of single-slab 3D turbo spin-echo imaging (SPACE, Siemens Healthcare, Erlangen, Germany) modified to support a stack-of-spirals acquisition. The signal evolution along the spin-echo train was mapped to the through-plane (3D) Cartesian phase-encoding direction to obtain the desired image-contrast properties3. In-plane acceleration was implemented by acquiring a reduced number of spiral interleaves (e.g., every second or third interleaf) for each k-space plane and reconstructing the resulting data using a SPIRiT4-based algorithm implemented on the MR scanner. The 3D data were first Fourier transformed along the third dimension, and SPIRiT-based reconstruction was then performed on each plane of undersampled spiral data. Dual-density spiral waveforms were used to provide fully sampled data for the central portion of each k-space plane and undersampled data for the remainder5. Trajectory corrections (gradient delay and eddy current) for each spiral interleaf were integrated into the online image reconstruction6.Typical parameters included a TR 2500 ms with a restore (driven equilibrium) “flip-back” RF pulse at the end of each spin-echo train, TE 610-850 ms, 16 dual-density 4480-µs spiral-out interleaves per k-space plane (3-fold acceleration), 140° refocusing RF pulses, in-plane spatial resolution 1.3 mm, through-plane spatial resolution 2.0 or 2.9 mm (interpolated to 2.0 mm), and 24 or 30 encoding steps in the 3D direction. Total acquisition (breath-hold) time was 18 seconds.
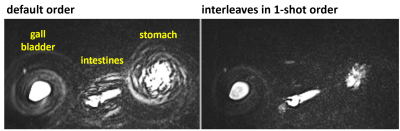
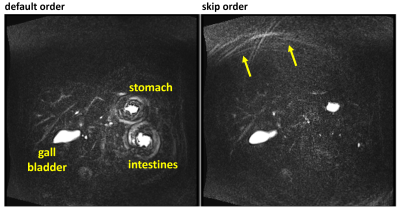
In some subjects, motion of fluids, such as in the stomach or intestines, occurred during the breath-hold period and caused artifacts. Four different encoding orders were evaluated with respect to artifact characteristics, including: (a) the flexible phase-encoding order used in the commercial SPACE pulse sequence7 adapted to map interleaf angles to echo trains such that the variation in interleaf angle among echoes was small for a given echo train8 (“default” encoding order, which collected all through-plane [Cartesian] encoding steps with each shot); (b) all spiral-interleaf angles collected in each shot with reordering of interleaves to suppress signal discontinuities (“interleaves in 1-shot” encoding order); (c) order b with, in addition, randomized order of through-plane Cartesian phase encoding (“shuffle” encoding order); and (d) order a with a large interleaf-angle increment between time-consecutive interleaves (“skip” encoding order).
The accelerated 3D stack-of-spirals turbo spin-echo acquisition and various encoding orders were tested in phantoms and in ten healthy volunteers (after obtaining informed consent) on 1.5T (MAGNETOM Aera) and 3T (MAGNETOM Prisma) MR scanners (both Siemens Healthcare, Erlangen, Germany) using phased-array body and spine RF coils.
Results & Discussion:
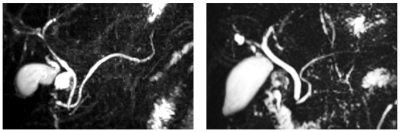
Figure 1, showing coronal maximum-intensity-projection images from two volunteers, illustrates the representative image quality obtained using the prototype 3D stack-of-spirals turbo spin-echo MRCP acquisition. The gall bladder and major ductal structures are depicted with high signal-to-noise ratio.Using the default encoding order, some subjects exhibited artifacts due to motion of fluids during the breath-hold period (Fig. 2, left). These in-plane swirl-like artifacts were suppressed using the interleaves in 1-shot encoding order (Fig. 2, right). Nonetheless, with the interleaves in 1-shot encoding order, the swirl-like artifacts were converted to ghost artifacts in the through-plane (Cartesian-encoding) direction (Fig. 3, left). The shuffle encoding order smeared out the Cartesian-ghost artifacts, but did not sufficiently decrease their intensity. The skip encoding order eliminated the Cartesian-ghost artifacts (Fig. 3, right) while moving the swirl artifacts toward the edge of the field of view and greatly reducing artifact intensity (Fig. 4, right). The use of variable (versus dual) density spirals may further reduce artifact intensity with the skip encoding order.
Conclusions & Future Work:
This preliminary study has demonstrated the feasibility of performing breath-hold MRCP using an accelerated 3D stack-of-spirals turbo spin-echo MRCP acquisition to obtain good image quality for evaluating major ductal structures. This method could be an alternative or adjunct to free-breathing methods in subjects with irregular breathing patterns. Several encoding orders were investigated for suppressing artifacts from fluid motion during the breath-hold period. Future work will include direct comparison of the 3D spiral-based technique to both free-breathing 3D MRCP and alternatives for breath-hold 3D MRCP, such as those described in references 1 and 2. We will also explore further optimization of the 3D spiral-based MRCP acquisition to permit shorter breath-hold durations, increased spatial resolution/coverage, and improved artifact suppression.Acknowledgements
No acknowledgement found.References
1. Chandarana H et al. Three-dimensional MR cholangiopancreatography in a breath hold with sparsity-based reconstruction of highly undersampled data. Radiology 2016; 280:585-94.
2. Nam JG et al. GRASE Revisited: Breath-hold three-dimensional (3D) magnetic resonance cholangiopancreatography using a Gradient and Spin Echo (GRASE) technique at 3 T. Eur Radiol 2018; 28:3721-3728.
3. Fielden S et al. Variable‐flip angle 3D‐turbo spin echo imaging utilizing spiral acquisitions. Proc ISMRM 19 (2011); 2820.
4. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med 2010; 64:457-471.
5. Meyer CH et al. Dual-density and parallel spiral ASL for motion artifact reduction. Proc ISMRM 19 (2011); 3986.
6. Tan H, Meyer CH. Estimation of k-space trajectories in spiral MRI. Magn Reson Med 2009; 61:1396-1404.
7. Li G et al. The shifted radial reordering for intermediate TE imaging in 3D long echo train acquisition. Proc ISMRM 17 (2009); 2623.
8. Li G et al. Reducing fluctuation of train trajectories in 3D TSE imaging with compressed sampling. Proc ISMRM 21 (2013); 3711.
Figures