2532
Placental Perfusion Imaging on Zika-Infected Rhesus Macaques using Velocity-Selective ASL MRI1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Wisconsin National Primate Research Center, University of Wisconsin - Madison, Madison, WI, United States, 3Comparative Biosciences, University of Wisconsin - Madison, Madison, WI, United States, 4Obstetrics & Gynecology, University of Wisconsin - Madison, Madison, WI, United States, 5Pathology, Oregon Health & Science University, Portland, OR, United States, 6Radiology, University of Wisconsin - Madison, Madison, WI, United States, 7Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Effective non-invasive assessment of placental health, particularly in early pregnancy, is of clinical interest but currently lacking. Arterial spin labeling (ASL) MRI can safely provide local functional assessment of placental perfusion, however, placental perfusion imaging is challenging and current approaches have shortcomings. Here we investigate a multi-slice velocity-selective (VS ASL) sequence with volumetric placental perfusion assessment and report on local and global perfusion across multiple gestational stages for zika-infected rhesus macaques and healthy controls.
Introduction
Abnormal placental perfusion is associated with pregnancy complications including fetal growth restriction and preeclampsia.1,2 Arterial Spin Labeling (ASL) perfusion MRI is a compelling method to safely and non-invasively assess local perfusion without the need for an exogenous contrast agent. However, ASL perfusion MRI in the placenta poses unique challenges, including (1) motion artefacts (fetus and maternal breathing motion), (2) variability in placental geometry and location, (3) maternal and fetal blood supply, and (4) long transit times from the arteries to intervillous space. FAIR ASL is limited to single slice acquisitions and pseudo-continuous (PC) ASL is commonly used in vascular regions such as the brain, but requires careful selection of tagging regions which is not conducive for placenta imaging due to the location and tortuous path of the feeding maternal arteries. Velocity-selective (VS ASL) MRI3 has emerged as an alternative approach where tagging is achieved based on its velocity instead of location and its feasibility for placenta imaging has been recently demonstrated for a 3D spiral sequence.4 Here we introduce a modified VS ASL sequence that provides volumetric coverage with an interleaved 2D multi-slice approach for reduced motion sensitivity and demonstrate its feasibility across gestational stages in a non-human primate model.Methods
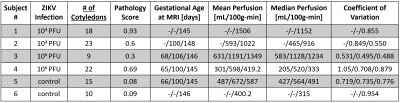
Subjects: Six rhesus macaques were imaged up to 3 times across gestational stages (Table 1). The subjects received a Zika virus (ZIKV) injection of 104 (2 subjects) or 106 (2 subjects) plaque forming units (PFU) and the control group (2 subjects) received a saline injection. All injections occurred in amniotic fluid around gestational age of 55 days (54.7±1.9 days).Pathology: After delivery via C-section, the placenta was analyzed for number of cotyledons and subsequently scored by a pathologist for abnormalities including chronic histiocytic intervillitis, acute chorioamninitis, placental infarction, and maternal decidual vasculitis (Table 1).
MRI: Scans were acquired at 3.0 T (Discovery MR750, GE Healthcare) with a 32-channel phased array coil. The maternal monkeys were anesthetized with isoflurane, thereby effectively eliminating fetal body motion, and imaged in right-lateral position. Standard SSFSE images were acquired for anatomy and placement of the VS ASL slices.
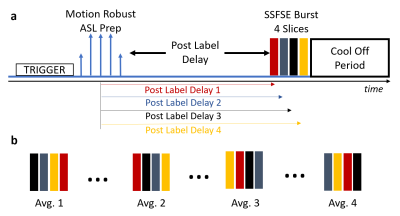
VS ASL Sequence: The newly developed interleaved 2D multi-slice VS ASL sequence is shown in Figure 1. It uses a 2D SSFSE multi-slice readout with slice interleaving to avoid a slice bias based on consistent differences in the post label delays. 4 slices were imaged in each shot to balance the increase in SAR and the longer acquisition window with varying post label delays. A cool off period of 2 s was added prior to the next excitation to limit SAR.
Symmetric, adiabatic BIR-8 preparation pulses with and without flow sensitizing gradients (TI=1.2s, TR=6.6s, venc=2.4cm/s) and fat saturation pulses were used for signal preparation. R/L motion encoding was used to reduce influence from maternal respiratory motion. Scan parameters: respiratory trigger (expiration); 2D SSFSE readout (TI = 1.2 s, TE=52.2ms, matrix=256x256, in-plane spatial resolution=1.6mm2, slice thickness 8 mm); 16 control/ tag pairs; 4 slices per shot; 2 sets of 4 interleaved slices for whole placenta coverage; scan time: ~7 min. In addition, a proton-density weighted (PDW) image without magnetization preparation was acquired in the beginning of the scan.
Processing: Offline image reconstruction and post-processing were performed using Matlab. Placental boundaries were manually segmented from PDW image using in-house software. Voxel-wise perfusion maps were generated for all 8 slices as the median of the 16 tag-on/off difference images, normalized by PDW. For each scan, the mean, median, and standard deviation across all data points in the placental perfusion map were calculated. The coefficient of variation is calculated as a ratio between mean and standard deviation. These values were also assessed for the central slice only.
Results
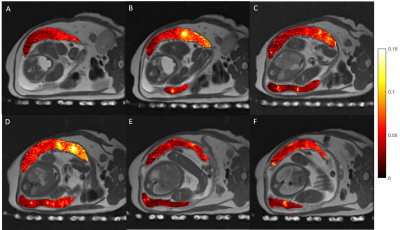
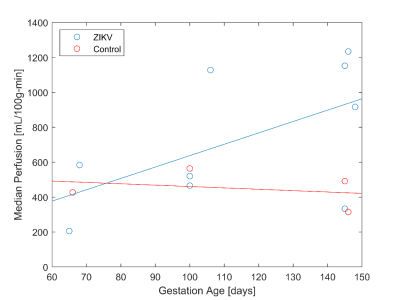
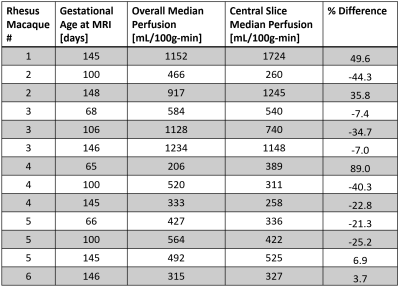
Tissue analysis showed that ZIKV subjects had an increase in number of cotyledons (with one exception) and higher pathology scores between 0.3 to 0.93 with varying pathology (not shown here). Perfusion maps were successfully obtained in all 13 scans. Figure 2 shows an example of six consecutive VS ASL maps indicating perfusion heterogeneity. Figure 3 shows the median VS ASL perfusion vs gestational age, which stays fairly constant for the controls and appears to increase with gestational age for ZIKV subjects. Table 4 compares the median perfusion using all placenta voxels vs central slice voxels only, demonstrating substantial variations.Conclusions
This study demonstrates the technical feasibility of 2D multi-slice VS ASL with whole placental coverage. Differences in pathology score, number of cotyledons, and perfusion changes over gestational age were observed between subjects injected with ZIKV and controls but more data are needed to allow for thorough statistical testing. Visual inspection and the difference in mean perfusion measures between the central slice and whole placenta in some subjects demonstrate the need for volumetric placenta analysis. It should be noted further noted that VS ASL MRI requires the adjustment of several soft parameters (VENC, TI, motion encoding direction, etc.) and the interpretation of results is currently not straight forward as the signal contains contributions from maternal and fetal blood and only selected velocity ranges, the implications of which need to be better understood before being useful in clinical imaging.Acknowledgements
We gratefully acknowledge GE Healthcare for research support and funding from the NIH Human Placenta Project (U01-HD087216) and NIH grant R21-AL129308.References
1 Siauve N, Gihad C, Benjamin D, Marianne A, Olivier C, Yves V, and Laurent JS. Functional imaging of the human placenta with magnetic resonance. American Journal of Obstetrics and Gynecology. 2015;213(4):103-114.
2 Hirsch, AJ et. al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nature Comm. 2018:9(263). doi:10.1038/s41467-017-02499-9
3 Wong EC, Cronin M, Wu W-C, Inglis B, Frank LR, and Liu TT. Velocity-selective arterial spin labeling. Magnetic Resonance in Medicine. 2006;55(6):1334-1341.
4 Zun Z. and Catherine L. Placental perfusion imaging using velocity-selective arterial spin labeling. Magnetic Resonance in Medicine. 2018;80(3):1036-1047.
Figures