2530
Fetal brain age estimation and anomaly detection using attention-based deep ensembles with uncertainty1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Key Laboratory of Biomedical Information Engineering of Ministry of Education, Department of Biomedical Engineering, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, China, 3Department of Radiology, Women's Hospital, School of Medicine, Zhejiang University, Hangzhou, China, 4School of Biomedical Engineering, Shanghai Jiaotong University, Shanghai, China
Synopsis
Accurate estimation of the brain age is important for the evaluation of brain development, especially in the fetal stage when little diagnostic tools are available. This study designed attention-based deep ensembles to estimate brain age in the normal developing fetus, based on axial T2-weighted in-utero MRI images from routine clinical scans. Mean absolute error of 0.803 week was achieved, and the attention maps highlighted the regions of interest associated with the estimation. Predictive uncertainty was simultaneously quantified, and together with the proposed prediction confidence, we were able to detect several types of anomalies, including small head circumference, malformations, and ventriculomegaly.
Introduction
MRI-based brain age estimation provides important markers in studies of brain development or aging1-3. However, the estimation of the fetal brain was not full-investigated despite its significance, partially due to the difficulty in utero MRI acquisition and the lack of large datasets4. In this study, we propose a learning-based technique for brain age prediction using routine clinical 2D multislice fetal brain MRI scans. Simultaneously, the predictive uncertainty can be quantified from the network to assess the morphologic abnormality in fetal brains. We trained an attention-based deep residual network and tested its performance in brain age estimation of the normal developing fetus, as well as its efficacy in detecting different types of fetal anomalies.Methods
Data acquisition: T2-weighted in-utero MRI scans of 665 healthy (GA: 22-39 weeks) and 50 abnormal (GA: 22-39 weeks) fetuses were retrospectively collected from the local hospital with IRB approval. The images were acquired on a 1.5 Tesla scanner (GE Signa HDxt) using a single-shot fast spin echo sequence with the following parameters: TE/TR=130/2400ms, FOV=360$$$\times$$$360mm, in-plane resolution=0.7mm, 9-20 slices with slice thickness of 3.0-4.5mm, axial orientation. The abnormal fetuses were divided into four groups, namely the large and small head circumference (HC), ventriculomegaly and malformation.Preprocessing: fetal brain was extracted using a U-Net based segmentation5, and then automatically cropped. The middle slice was designated as the slice with the largest brain area. Each slice was resized into 192×192 by padding zero in the surrounding regions and was then realigned and normalized. Altogether, seven slices (three slices inferior and superior to the middle slice) were taken into account.
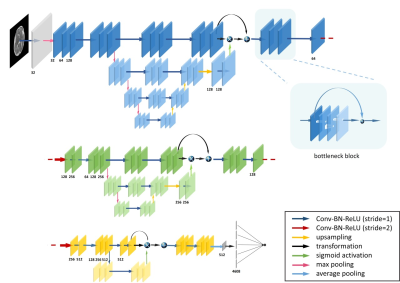
Network details: Different training strategies were evaluated. Three types of input schemes, including the single-slice (middle slice), 2D multi-slice, and 3D volume, were compared. The attention mechanism was then added to the optimal scheme to enhance prediction6,7. The network was constructed by three different attention modules, each of which contained a truck branch and a mask branch (Figure 1). The first two mask branches adopted the bottom-up and top-down structure, integrating multi-scale information using shortcuts8. The third module adopted features from higher layers to acquire the attention maps with better semantic abstraction9. Moreover, the uncertainty was modeled by assuming the data $$$\mathcal{D}\text{= }\!\!\{\!\!\text{ }\mathbf{x}\text{ }\!\!\}\!\!\text{ }_{n=1}^{N}$$$ follows a heteroscedastic Gaussian distribution $$$\mathcal{N}(y;\mu (\mathbf{x},\mathbf{w}),{{\sigma }^{2}}(\mathbf{x},\mathbf{w}))$$$, where x, $$$y$$$, w denotes the MR images, gestational age and model weights, respectively. The model was trained by minimizing negative log-likelihood $$$\mathcal{L}(\mathsf{\mathcal{D}})$$$, and the predictive uncertainty $$$\delta (\mathbf{x})$$$ and predicted brain age $$$\mu (\mathbf{x})$$$ were therefore computed via network ensembles10,11. $$\mathcal{L}(\mathsf{\mathcal{D}})=\sum\limits_{i}{\frac{{{({{y}_{i}}-\mu ({{\mathbf{x}}_{i}},\mathbf{w}))}^{2}}}{2{{\sigma }^{2}}({{\mathbf{x}}_{i}},\mathbf{w})}}+\frac{1}{2}\sum\limits_{i}{\log }{{\sigma }^{2}}({{\mathbf{x}}_{i}},\mathbf{w}) $$ Then, a robustly estimated confidence $$$c$$$ of predicted brain age is proposed.$$c(\mathbf{x},y)=\mathcal{N}(y;\hat{\mu }(\mathbf{x}),\hat{\delta }(y))$$ GA-corrected absolute age difference (AAD), predictive uncertainty and confidence of brain age were used to differentiate different types of abnormal fetuses from the normal ones.
Results
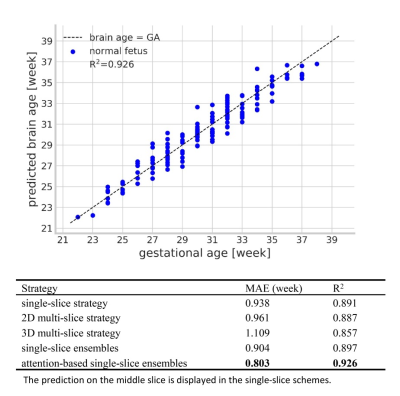
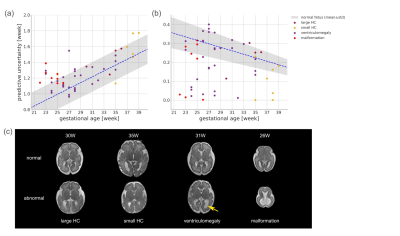
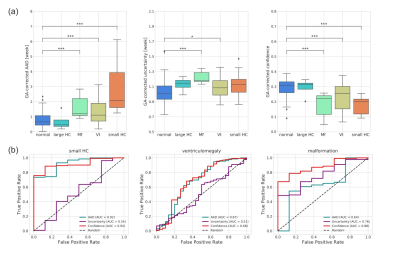
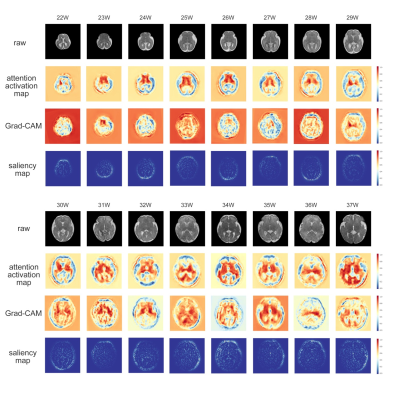
The network trained in single-slice strategy achieved better performance than multi-slice schemes, and the mean absolute error (MAE) for deep ensembles on the middle slice was 0.803 week and the corresponding R2 was 0.926 for the attention-driven deep ensembles (Figure 2). The predictive uncertainty showed a strong positive linear correlation over gestational age (Figure 3A), while the confidence of fetal brain age showed a moderate negative dependence (Figure 3B). As the uncertainty plotted on top of the normal curves, it was observed that the abnormal data had higher predictive uncertainty and lower confidence of brain age than those of healthy fetuses (gray shades).Figure 4A demonstrated that the brain ages of the abnormal fetus were beyond the normal range according to the AAD, except the fetuses with large HC, and the predictive uncertainty significantly increased in the fetus with malformations. Moreover, the predicted brain ages of abnormal fetus were significantly less convinced than the healthy groups. The classification results revealed the confidence of brain age had good capability in detecting different types of anomalies while AAD showed the best performance in detecting the fetuses with small HC over the other markers (Figure 4B). Additionally, attention activation maps and Grad class activation maps in the first module and saliency maps of inputs were obtained to visualize the regions associated with fetal age prediction (Figure 5). The margins between gray matters and cerebrospinal fluid were highlighted indicating these regions were critical during fetal brain development.
Discussion and Conclusion
In this study, we developed an attention-based residual network based on model ensembles with uncertainty to estimate the brain age of fetus, using axial MR scans only. It achieved an accurate prediction, and the uncertainty was simultaneously quantified for anomaly detection. The predictive uncertainty was less sensitive to the size of the fetal brain, but it has a good sensitivity to malformations. Additionally, the confidence of brain age was proposed by combining the predicted age error and predictive uncertainty, which showed a promising capability in detecting several types of fetal anomalies. Future work will be focused on adding prior information into the network training for better prediction and detection and employing patch-based fetal brain age estimation.Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Kaufmann T, van der Meer D, Doan N T et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature neuroscience. 2019; 22(10):1617-1623.
2. Cole J H, Franke K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends in neurosciences. 2017; 40(12):681-690.
3. Franke K, et al. Brain maturation: predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 2012; 63(3): 1305-1312.
4. Studholme C. Mapping fetal brain development in utero using magnetic resonance imaging: the Big Bang of brain mapping. Annual review of biomedical engineering, 2011;13:345-368.
5. Ronneberger O, Fischer P, and Brox T. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention, 2015; 234-241.
6. Vaswani, A, et al. Attention is all you need. Advances in neural information processing systems. 2017; 5998-6008
7. Hu J, Shen L. and Sun G., 2018. Squeeze-and-excitation networks. Proceedings of the IEEE conference on computer vision and pattern recognition. 2018; 7132-7141.
8. Wang F, Jiang M, Qian C, et al. Residual attention network for image classification. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2017; 3156-3164.
9. Zhang J, Xie Y, Xia Y, et al. Attention residual learning for skin lesion classification. IEEE transactions on medical imaging, 2019.
10. Lakshminarayanan B, Pritzel A, Blundell C. Simple and scalable predictive uncertainty estimation using deep ensembles. Advances in Neural Information Processing Systems. 2017; 6402-6413.
11. Beluch W H, Genewein T, Nürnberger A and Köhler J M. The power of ensembles for active learning in image classification. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2018; 9368-9377.
Figures