2522
MRI findings of uterine adenomatoid tumors including diffusion-weighted imaging with pathologic correlation
Mayumi Takeuchi1, Kenji Matsuzaki2, and Masafumi Harada1
1Department of Radiology, Tokushima University, Tokushima, Japan, 2Department of Radiological Technology, Tokushima Bunri University, Sanuki-city, Japan
1Department of Radiology, Tokushima University, Tokushima, Japan, 2Department of Radiological Technology, Tokushima Bunri University, Sanuki-city, Japan
Synopsis
Uterine adenomatoid tumor (AT) is a rare benign neoplasm of mesothelial origin. MRI findings of surgically proven 10 ATs were retrospectively evaluated. Most ATs (9/10) appeared as a heterogeneous intensity mass on T2WI with admixture of well-defined myoma-like low intensity area reflecting the areas of smooth muscle hypertrophy, and ill-defined high intensity areas reflecting the areas of abundant tubules. AT may contain high intensity areas on DWI, occasionally appearing as ring-like high intensity area (3/9), with relatively high ADC (6/9) due to T2 shine-through effect. Intra-tumoral hemorrhage was observed on SWAN (1/6) but not on T1WI (0/10).
Introduction
Adenomatoid tumor (AT) is a rare benign genital tract neoplasm of mesothelial origin, and commonly affects fallopian tubes and the uterus. AT arising from uterus typically occurs in women of reproductive age, and is found in 1-5% of hysterectomy specimens. AT is usually located subserosal or in the outer myometrium, and typically a small solid mass but occasionally larger, or may be cystic1-4. Most of uterine AT may be misdiagnosed as leiomyoma because it shared the same imaging features of leiomyoma5-7. Because leiomyoma is sensitive for hormone therapy, unnecessary hormone therapy may be administered for AT which is misdiagnosed as leiomyoma. In addition, because AT is densely adherent to the surrounding myometrium without separating layer or capsule, it may be difficult to enucleate and surgeons should modify the procedure during laparoscopic “myomectomy”8, 9. Therefore, it is important to differentiate AT from leiomyoma for the adequate treatment. The purpose of this study is to evaluate the MRI findings of AT including DWI for the differentiation from leiomyoma.Methods
Pathologically proven 10 ATs in 9 women (29-54 years of age, mean 42 years) who had undergone MRI examinations with 3T (2 lesions in 2 cases) or 1.5T (8 lesions in 7 cases) superconducting MRI systems before surgery were retrospectively evaluated (Fig. 1). Fast spin-echo T2WI and spin-echo or gradient-echo T1WI with/without fat-saturation were obtained in all 9 patients. Gadolinium-enhanced T1WI were obtained in 7 lesions in 6 cases. DWI (b=800s/mm2) was obtained in 9 lesions. Susceptibility-weighted sequence (SWAN) was obtained in 6 lesions. Two gynecologic radiologists both with more than 20-years experiences qualitatively evaluated the images: location, shape, size, margin, signal intensity on T1WI (plain/CE), T2WI, DWI (b=800), and computed DWI (b=1500); the presence of hemorrhagic foci (high intensity on T1WI and signal voids on SWAN). The reviewers examined all images of the cases independently and then resolved discrepancies by consensus. The mean ADC values were measured in 3 circular ROIs within a mass (entire mass, high intensity area and low intensity area on DWI, respectively) from ADC maps generated by using b-values of 0 and 800 on the workstation (AW4.2).Results and Discussions
All 10 tumors exhibited as subserosal or outer-myometrial round solid masses, and no predominantly cystic mass was observed. The maximum diameter of the tumor was 2.2 to 5.0 cm (mean, 3.3 cm). Nine of 10 lesions showed heterogeneous signal intensity with admixture of partially ill-defined high signal intensity areas and well-defined myoma-like low signal intensity areas on T2WI. The other one lesion showed slight low signal intensity with totally ill-defined margin on T2WI. Ring-like high intensity area was observed on T2WI in 4 ATs. Because AT is composed of mesothelial origin tubules of varying size with smooth muscle hypertrophy (Fig. 2), areas containing small cystic dilatations of abundant tubules may increase the signal intensity on T2WI, whereas areas of smooth muscle hypertrophy may show low signal intensity like leiomyoma3, 4. In our study, the admixture of areas of smooth muscle hypertrophy showing low signal intensity with well-defined margins, and areas of abundant tubules showing high signal intensity with ill-defined margins on T2WI may be characteristic for AT reflecting its pathological features (Fig. 3, 5). Areas of abundant tubules showed high signal intensity on DWI (b=800) and signal decrease on computed DWI (b=1500) with relatively high ADC (high intensity areas: 1.4 to 1.73, mean 1.54 x 10-3 mm2/s; low intensity areas: 1.14 to 1.43, mean 1.33 x 10-3 mm2/s) in 6 of 9 lesions suggesting T2 shine-through effect (Fig. 4, 5). Ring-like high intensity area was observed on DWI in 3 ATs, which may be rarely observed in leiomyomas. In the other 3 lesions, 2 lesions did not contain high signal intensity areas on DWI, and one lesion showed high signal intensity both on DWI and computed DWI (b=1500) with relatively low ADC (1.14) suggesting water diffusion restriction. However uterine myometrial mass with ill-defined margins and/or with high intensity areas on T2WI and DWI may have a probability of malignancy such as sarcomas, however, relatively high ADC of most ATs may be suggestive for its benignity. High intensity hemorrhagic foci on T1WI were not observed in all 10 lesions. SWAN showed small signal voids reflecting hemorrhage in one lesion with hyalinized necrosis possibly due to partial ischemic changes. Intra-tumoral hemorrhagic necrosis is suggestive finding for uterine sarcomas10. In this study, most ATs showed no intra-tumoral hemorrhage, which may be compatible with benign tumor. Co-existing leiomyomas were demonstrated in 6 of 9 patients. Degree of enhancement was either lower than (n=6) or equal to that of the myometrium (n=1). Relatively weak contrast-enhancement of ATs may be due to unenhanced small cystic spaces formed by the dilatation of abundant tubules (Fig. 5).Conclusion
We conclude that typical AT may appear as a heterogeneous signal intensity mass containing myoma-like low signal intensity areas with well-defined margins and high intensity areas with ill-defined margins on T2WI. AT may contain high signal intensity areas on DWI, occasionally appearing as ring-like high intensity area, with relatively high ADC due to T2 shine-through effect reflecting abundant tubules. Intra-tumoral hemorrhage may also be rare on MRI.Acknowledgements
No acknowledgement found.References
- Nogales FF, et al. Adenomatoid tumors of the uterus: an analysis of 60 cases. Int J Gynecol Pathol. 2002;21:34-40.
- Sangoi AR, et al. Adenomatoid tumors of the female and male genital tracts: a clinicopathological and immunohistochemical study of 44 cases. Mod Pathol. 2009;22:1228-1235.
- Prat J, et al. Tumours of the uterine corpus: Miscellaneous tumours: Adenomatoid tumour. In: Kurman RJ (Eds.) WHO Classification of Tumours of Female Reproductive Organs, 4th ed., World Health Organization, 151, 2014.
- Oliva E, et al. Mesenchymal tumors of the uterus: Miscellaneous mesenchymal tumors and conditions: Adenomatoid tumor. In: Kurman RJ (Eds.) Blaustein's Pathology of the Female Genital Tract, 7th ed., Springer, 621-2, 2019.
- Mitsumori A, et al. MR appearance of adenomatoid tumor of the uterus. J Comput Assist Tomogr. 2000;24:610-613.
- Kim JY, et al. Cystic adenomatoid tumor of the uterus. AJR. 2002;l179:1068-1070.
- Meng Q, et al. Magnetic Resonance Imaging and Pathologic Findings of 26 Cases With Uterine Adenomatoid Tumors. J Comput Assist Tomogr. 2015; 39:499-505.
- Alkatout I, et al. Precarious preoperative diagnostics and hints for the laparoscopic excision of uterine adenomatoid tumors: two exemplary cases and literature review. Fertil Steril. 2011;95:1119.e5-8.
- Sakurai N, et al. Laparoscopically resected uterine adenomatoid tumor with coexisting endometriosis: case report. J Minim Invasive Gynecol. 2011;18:257-261.
- Takeuchi M, et al. Clinical utility of susceptibility-weighted MR sequence for the evaluation of uterine sarcomas. Clin Imaging. 2019;53:143-150.
Figures
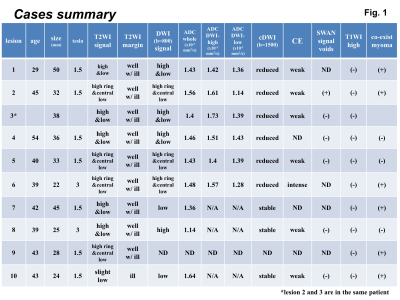
Cases summary: Clinical data and MRI findings of the cases were summarized.
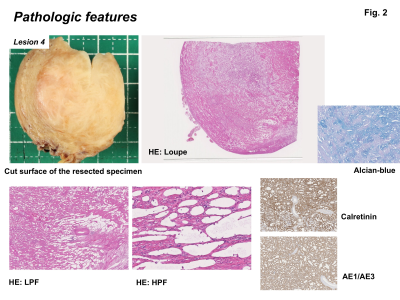
Pathologic features: AT have relatively ill-defined borders compared with leiomyoma, with a nodular, whitish firm cut-surface. AT is composed of variably sized tubules and associated with smooth muscle hypertrophy. Alcian-blue positive material is seen within lumens. Tumor cells express AE1/AE3 and calretinin.
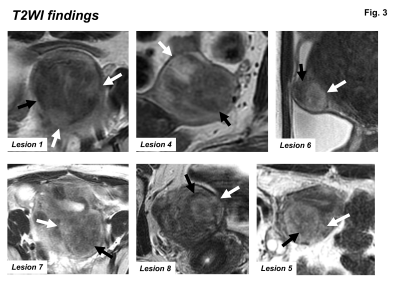
T2WI findings: Most ATs showed heterogeneous signal intensity on T2WI, containing myoma-like low signal intensity portion with relatively well-defined margins (black arrows) and high signal intensity portion with ill-defined margins (white arrows).
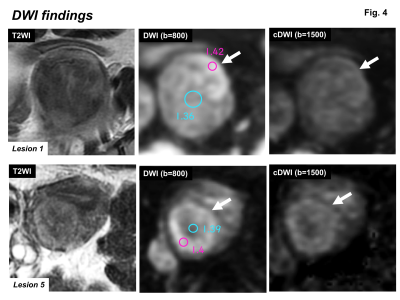
DWI findings: Most ATs contained high signal intensity area (arrows) on DWI (b=800 s/mm2), which showed signal decrease on computed DWI (b=1500 s/mm2) with relatively high ADC suggesting T2 shine-through effect. Ring-like high intensity area on DWI was observed in some lesions.
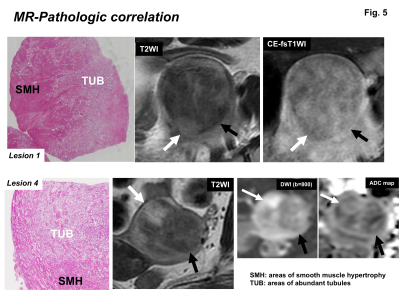
MR-Pathologic correlation: Areas with abundant tubules (white arrows) showed high signal intensity on T2WI due to T2 prolongation, high signal intensity on DWI (b=800 s/mm2) due to T2 shine-through effect, and relatively weak contrast-enhancement due to unenhanced small cystic spaces. Whereas areas of smooth muscle hypertrophy showed low signal intensity on T2WI and DWI.