2513
PANCREATIC IRON IN PATIENTS WITH HEMOCHROMATOSIS: NOT AS RARE AS CARDIAC IRON1Diagnostic and Interventional Radiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 2Biochemistry, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 3Diagnsotic and Interventional Radiology, Universitätsmedizin Charité, Berlin, Germany, 4Hemato-Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5Diagnostic Radiology, UCSF-Benioff Children's Hospital Oakland, Oakland, CA, United States
Synopsis
HFE-associated hereditary Hemochromatosis (HFE-HH) is the most frequent monogenic genetic disorder in the Caucasian population. The excessive iron storage in organs are common, affecting the liver mostly (> 90 %) resulting in a potentially severe liver damage (fibrosis, cirrhosis). In recent years, hepatic and cardiac iron deposition has been studied in detail. Due to its involvement in the development of diabetes - a frequent co-morbidity in iron overload diseases - pancreatic iron should become a field of interest. This study aims to determine the pancreatic iron and fat content in patients with HFE-HH.
Introduction
Fast quantitative magnetic resonance imaging (qMRI) methods will facilitate more systematic studies of iron accumulation in different organs. Unclear at the moment is the relevance of non-liver iron storage in different diseases with iron overload. What is known in subjects under chronic transfusion treatment (e.g. ß-thalassemia major) is the clinical relevance of a life-threatening heart iron overload. In all iron overload diseases, the majority of the excess iron is stored in the liver and the SQUID-BLS-measurements of LIC is a valuable noninvasive techniques, proven in practise), to precisely quantify LIC in all kind of patients (3-89 years).HFE-associated hereditary Hemochromatosis (HFE-HH) is the most frequent monogenic genetic disorder in the Caucasian population. Due to a disturbed hepcidin-ferroportin axis, the food iron absorption is permanently increased leading to an excessive iron storage in organs. The liver is mostly affected (> 90 %) resulting in a potentially severe liver damage (fibrosis, cirrhosis). The exact distribution of iron into other organs is not well established and may also be variable among affected patients. In recent years, hepatic and cardiac iron deposition has been studied in detail. Due to its involvement in the development of diabetes - a frequent co-morbidity in iron overload diseases - pancreatic iron should become a field of interest. This study aims to determine the pancreatic iron and fat content in patients with HFE-HH.
Material and Methods
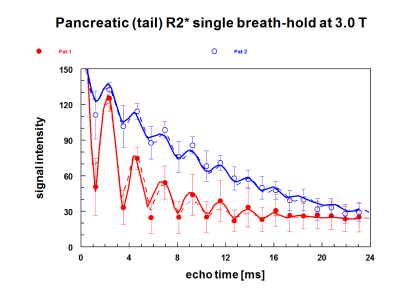
In this study we used biomagnetic liver susceptometry(BLS) for measuring liver iron (LIC) and quantitative MRI using multiple gradient-echo sequences (echo time 1.3–25.7 ms) for the determination of transverse relaxation rates R2* (=1/T2*). In the heart, septal R2* was determined from signal intensities by a mono-exponential model, while pancreatic iron (R2*) and fat was assessed by fat-water chemical shift relaxometry. So far, these measurements were performed in 21 patients with HFE-HH in a wide range of iron loading.Results
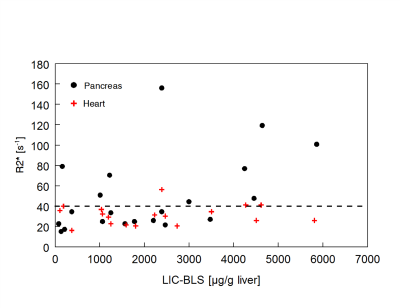
As shown in Fig.1, the presence of heart iron is rare in patients with HFE-HH even in severely iron loaded patients. Among about 500 patients with HFE-HH, we have seen only one patient with very severe iron overload presenting clinically impressive heart failure which recovered very quickly under DFO and phlebotomy treatment (Nielsen et al. BJH 2003, 123, 952–953).As seen from the R2*-rates in the pancreas, the pancreas, especially the tail, is more frequently affected in severely loaded subjects but also in some milder cases. A larger prospective study must be awaited to document a pancreatic risk also in HFE-HH patients under routine diagnostic and treatment procedure.
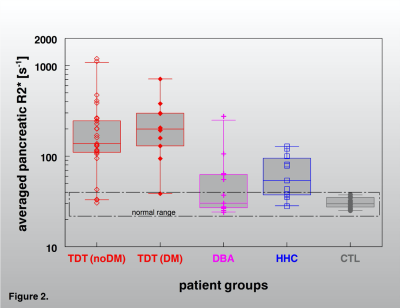
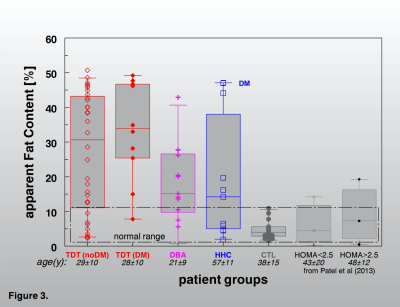
Judged by the R2*-rates in the pancreas tail, this organ is more frequently affected by excessive iron storage mainly in severely loaded subjects with HFE-HH but also in some milder cases. The comparison with other iron overload diseases (Fig. 4 and 5) shows that pancreatic iron overload and a high pancreatic fat content may also be a relevant risk factor in patients with in hemochromatosis. However, a larger prospective study must be awaited to document a pancreatic risk also in HFE-HH patients under routine diagnostic and treatment procedure.
Conclusion
Pancreatic iron overload and a high pancreatic fat content may also be a relevant risk factor in patients with in hemochromatosis. However, a larger prospective study must be awaited to document a pancreatic risk also in HFE-HH patients under routine diagnostic and treatment procedure.Acknowledgements
No acknowledgement found.References
S.N Rasmussen. British Journal of Radiology1972, 45, 579−585
K.E Brown et al. Translational Research 2006;148:55−62.
M.B Troadec et al. J Hepatol 2006; 44: 391–399.
Figures