2504
Free-Breathing, Confounder-Corrected T1 Mapping in the Liver with 3D Radial Inversion Recovery MRI1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Waisman Center, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Quantitative T1 mapping in the liver is an emerging biomarker of hepatic fibrosis and characterization of liver function. Existing T1 mapping methods in abdomen are generally sensitive to tissue fat and B1 inhomogeneities , both of which confound estimates of T1. Further, Cartesian methods may suffer from motion related ghosting artifacts. In this work, we propose to combine 3D-radial inversion recovery with chemical shift encoded imaging to jointly estimate T1 of water, T1 of fat, proton density fat fraction (PDFF), and B0 and B1 inhomogeneities. The feasibility and performance of the proposed method are evaluated with simulations, and phantom experiments.
Introduction
Quantitative T1 mapping is an emerging quantitative biomarker of hepatic fibrosis1,2,3 and liver function4. Conventional methods for T1 mapping in the liver include variable flip angle strategies with additional multi-echo spoiled gradient echo (SGRE) acquisitions to account for the tissue fat5,6. However, VFA methods are sensitive to B1 inhomogeneities and require a separate calibration scan to correct the estimation errors7. Furthermore, Cartesian sampling trajectories are limited to breath-holding approaches to avoid motion related ghosting artifacts, which may limit their use in children, the elderly and sick patients.Therefore, the purpose of this work is to develop and validate a novel method for T1 mapping in the liver, that takes advantage of motion insensitivity of 3D-radial acquisition and is capable of addressing multiple confounding factors. The proposed method combines 3D-radial inversion recovery and multi-echo SGRE sequences with variable flip angles and echo times to simultaneously estimate T1 of water (T1w), T1 of fat (T1f), proton density fat fraction (PDFF), and B0 and B1 inhomogeneities using a multi-compartment fitting strategy.
Theory
Inversion recovery-based acquisitions are popular for T1 mapping strategies due to the excellent dynamic range in T1 recovery created by inversion of the longitudinal magnetization. A recently published work by Kecskemeti et al. described an efficient way of collecting an arbitrarily large number of T1 weighted images using a 3D-radial inversion recovery sequence8 called ”MPnRAGE”. This method was shown to be capable of obtaining 3D volumetric T1 maps in the brain. Here, we extend this method to acquire multi-echo SGRE images to account for effects of tissue fat. In this work, we use a 2-compartment signal model for fat and water (each with independent T1 relaxation, inversion pulse efficiency, and initial phase), while fitting for a 6-peak fat9. Assuming perfect spoiling between each excitation RF pulse, we can describe the signal equation as follows:$$S_{w,MPnRAGE}(TI,TR,TD,\alpha;T_{1,w},\kappa_1,\kappa_{2,w})=((1-E_{R,w})\cdot~\frac{1-a_w^n}{1-a_w}+((1-E_{I,w})+M_{SS,w}(E_{D,w})\cdot~cos(\kappa_{2,w}\cdot~180)\cdot~E_{I,w})\cdot~a_w^n)\cdot~sin(\kappa_1\cdot~\alpha)$$
$$S_{f,MPnRAGE}(TI,TR,TD,\alpha;T_{1,f},\kappa_1,\kappa_{2,f})=((1-E_{R,f})\cdot~\frac{1-a_f^n}{1-a_f}+((1-E_{I,f})+M_{SS,f}(E_{D,w})\cdot~cos(\kappa_{2,f}\cdot~180)\cdot~E_{I,f})\cdot~a_f^n)\cdot~sin(\kappa_1\cdot~\alpha)$$
$$S_{w,SGRE}(TE;\theta_w,\Delta B_0,T_2^*,\rho_w)=\rho_w\cdot~e^{-TE/T_2^*}\cdot~e^{i(\theta_w+2\pi~f_0TE)}$$
$$S_{f,SGRE}(TE;\theta_f,\Delta B_0,T_2^*,\rho_f)=\rho_f\cdot~e^{-TE/T_2^*}\cdot~e^{i(\theta_f+2\pi~f_0TE)}\cdot~\sum_{p=1}^P m_p\cdot~e^{i2\pi f_pTE}$$
$$E_{R(w,f)}=e^{-TR/T_{1(w,f)}},E_{I(w,f)}=e^{-TI/T_{1(w,f)}},E_{D(w,f)}=e^{-TD/T_{1(w,f)}},a_{(w,f)}=cos(\kappa_1\cdot \alpha)\cdot E_{R(w,f)}$$
$$S_{combined}=S_{w,MPnRAGE}\cdot~S_{w,SGRE}+S_{f,MPnRAGE}\cdot~S_{f,SGRE}$$
Where, subscripts 'w' and 'f' denote signal parameters for water and fat signals, 'n' denotes the index of the excitation pulse in the inversion recovery sequence, $$$M_{SS,(w,f)}$$$ denotes the longitudinal magnetization at the end of one inversion recovery cycle, $$$\rho_{w,f}$$$ denotes signal amplitudes, $$$\theta_{w,f}$$$ denotes initial phase, $$$\kappa_1$$$ and $$$\kappa_{2(w,f)}$$$ denote efficiencies of excitation and inversion RF pulses (note for adiabatic pulses, due to duration of the pulse efficiency may differ between fat and water), $$$f_0$$$ denotes the frequency shift due to B0 inhomogeneity.
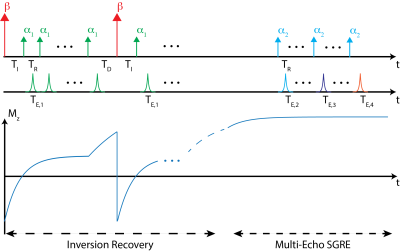
A diagram of the proposed pulse sequence is given in Figure 1, where $$$\beta$$$ denotes the inversion and $$$\alpha$$$ denotes the excitation pulses. TEi denotes the echo time corresponding to Ith SGRE acquisition.
Methods
Numerical SimulationsThe proposed acquisition and 2-compartment joint fitting method were tested using simulation data over a wide range of physiologically relevant liver T1w (300-1100ms) and PDFF (0-50%) values. Other simulation parameters include: $$$T_2^*=15ms$$$, $$$\kappa_1=0.95$$$, $$$\kappa_{2w,f}=0.87$$$, $$$T_{1f}=270ms$$$, $$$\Delta~B0=1ppm$$$. Acquisition parameters are chosen as follows, $$$\beta=180^o$$$,$$$\alpha_1=4^o$$$, $$$\alpha_2=8^o$$$, TE1=0.86ms, TE2=1.12ms, TE3=1.52ms, TE4=2.32ms, TR=5ms, TI=15ms, TD=500ms (see fig.1), SNR=25. Since signal amplitude varies over the acquisition, SNR is defined as maximum signal amplitude in the acquired signal divided by the standard deviation of the noise.
Phantom Experiments
Two sets of phantoms experiments are presented to demonstrate validation of the T1 mapping accuracy and feasibility of correction for tissue fat.
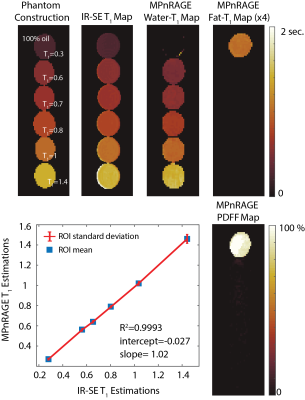
For the validation study, a single compartment phantom, consisting of 5 doped water vials with varying T1 values and one oil vial, was used. Since single compartment nature of the phantom allows for a magnitude fitting without correction of partial volume effects, this phantom was also scanned with an inversion recovery spin echo (IR-SE) sequence at varying TIs to provide a reference measurement.
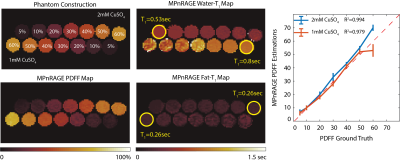
For the feasibility study, a phantom consisting of varying fat fractions (5-60%) and 2 different T1w values (obtained by addition of various amount of CuSO4 salt) was used. Imaging was performed with the high density, multi-channel body coil on a 3T system (AIR Coil, GE Healthcare Signa Premier, Waukesha, WI).
Results
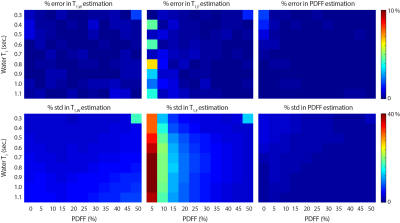
Numerical SimulationsMonte-Carlo simulations demonstrated the proposed strategy converges to unbiased estimators for all parameters of interest. Figure 2 shows the median and standard deviations of estimated T1w, T1f and PDFF.
Phantom Experiments
Validation of the proposed method is shown in Figure 3, where, estimated T1w and T1f values show linear agreement with IR-SE acquisitions.
Feasibility for fat-correction of the proposed method is demonstrated by joint estimation of PDFF and T1f in Figure 4. Estimated T1w values showed good agreement with CuSO4 concentrations, and PDFF values showed linear agreement with ground truth values.
Discussion
In this work, we have successfully presented a novel acquisition and fitting strategy for T1 mapping in the liver, that can jointly estimate and correct for multiple confounding factors including B0 and B1 inhomogeneities and tissue fat. This strategy combines an inversion-recovery based T1 mapping technique (MPnRAGE) and multi-echo SGRE to modulate T1, T2* contrasts and spin phase for simultaneous estimation of multiple confounding parameters. Future work will focus on improving the acquisition strategy to increase efficiency and account for T2* effects, and optimization of acquisition parameters.Acknowledgements
We wish to acknowledge support from the NIH (R01 DK083380, R01 DK088925, K24 DK102595, UL1TR002373) and from the University of Wisconsin SEED grant program. Further, we wish to acknowledge GE Healthcare and Bracco Diagnostics who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Banerjee R et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol, 60(1):69 – 77, 2014.
2. Haimerl M et al. Assessment of clinical signs of liver cirrhosis using t1 mapping on gd-eob-dtpa-enhanced 3t mri. PLOS ONE, 8(12):1–8, 12 2014.
3. Hoad CL et al. A study of t1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed, 28(6):706–714, 2015.
4. Kim JE et al. T1 mapping for liver function evaluation in gadoxetic acid–enhanced mr imaging: comparison of look-locker inversion recovery and b1 inhomogeneity-corrected variable flip angle method. European Radiology, 29(7):3584–3594, Jul 2019.
5. Tamada D et al. Multiparameter estimation using multi-echo spoiled gradient echo with variable flip angles and multicontrast compressed sensing. Magn Reson Med, 80(4):1546–1555, 2018.
6. Wang J et al. T1-corrected quantitative chemical shift-encoded mri (in press). Magn Reson Med, 2019.
7. Hai-Ling Margaret Cheng and Graham A Wright. Rapid high-resolution t1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med, 55(3):566–574, 2006.
8. Kecskemeti S et al. Mpnrage: A technique to simultaneously acquire hundreds of differently contrasted mprage images with applications to quantitative t1 mapping. Magn Reson Med, 75(3):1040–1053, 2016.
9. Hamilton G et al. In vivo characterization of the liver fat 1h mr spectrum. NMR Biomed, 24(7):784–790, 2011.
Figures