2487
A New Possibility of Late Phase Image in Gd-EOB-DTPA-enhanced MRI: Visual Assessment of Hepatic Function and Fibrosis Based on Uptake Rate of EOB1Radiology, Edogawa Hospital, Tokyo, Japan
Synopsis
We identified visual assessment value of late phase image to assess abnormal hepatic function and fibrosis. We retrospectively selected 41 patients who underwent Gd-EOB-DTPA enhanced MRI and classified them into three groups based on visual assessment of late phase images. Kruskal–Willis t-test was used to assess significant differences in the LSR of hepatobiliary phase, FIB-4, APRI, and platelet count. Intra-class correlation coefficient (ICC) was used for intra-reader visual assessment of late phase image. Significant differences in all parameters were observed in the groups. ICC was 0.85. Hepatic function or fibrosis might be assessed by visual assessment of late phase image.
Introduction
Gd-EOB-DTPA-enhanced MRI is a useful method to assess hepatic function and fibrosis and to detect intrahepatic tumors. This non-invasive technique determines the degree of hepatic function and fibrosis by quantifying the Gd-EOB-DTPA selective hepatic uptake agent in the normal and abnormal hepatocytes.1-5 Gd-EOB-DTPA hepatocyte-specific uptake has been reported to start 90 seconds following the injection of the contrast material, whereas the hepatic parenchyma is strongly and densely stained after approximately 10 minutes.6 Furthermore, Kessel et al.7 reported that normal hepatocytes exhibit significantly faster Gd-EOB-DTPA uptake than abnormal ones. Gd-EOB-DTPA dynamic imaging is constructed by means of arterial, portal, late, and hepatobiliary phase imaging.6 The dynamic imaging of arterial, portal, and late phases provides intrahepatic blood flow information, whereas hepatobiliary phase imaging can facilitate the detection of intrahepatic tumors and assessment of hepatic function and fibrosis.1-5 However, during the late phase of Gd-EOB-DTPA, it shows different the properties against equilibrium phase in conventional extracellular fluid contrast agent because Gd-EOB-DTPA gradually begins to migrate to the hepatocytes. Therefore, it does not necessarily reflect the equilibrium of extracellular fluid.6 Consequently, the aim of the study is to investigate the feasibility for hepatic function and fibrosis visual assessment using late phase imaging based on the uptake process of Gd-EOB-DTPA.Methods
Our institutional review board approved this retrospective study and waived the requirement of obtaining informed consent due to the retrospective nature of the study. We retrospectively selected 41 consecutive patients who underwent Gd-EOB-DTPA enhanced MRI examination at 1.5 Tesla for intrahepatic lesion evaluation from April 2018 to August 2019. All patients received intravenous administration of 0.1 ml/kg body weight of Gd-EOB-DTPA (Signa HDx; GE Healthcare, Chicago, IL), eight-channel body coil (Table 1). Data were classified into three groups defined by the signal intensity (SI) difference in the hepatic vein against that of the hepatic parenchyma as follows: high SI group: hepatic vein SI greater than the hepatic parenchymal SI; iso SI group: hepatic vein SI equal to the hepatic parenchymal SI; and low SI group: hepatic vein SI lower than hepatic parenchymal SI (Fig 1). We evaluated whether significant differences in the liver to spleen ratio (LSR) of hepatobiliary phase related to hepatic function and whether biochemical markers (FIB-4, APRI, and platelet count) related to hepatic fibrosis among the three groups using the Kruskal–Wallis t-test. Hematological examinations were derived within 1 week before and after MR imaging. Furthermore, the inter-reader reproducibility of the visual assessment on late phase images between two readers (readers 1 and 2: radiology technicians with 13 and 8 years of experience in MRI, respectively) was evaluated using the intra-class correlation coefficient (ICC).Results
The visual assessment results were as follows: high, iso, and low SI groups included 8, 13, and 20 patients, respectively (Table 2). Significant differences were observed in LSR, FIB-4, APRI, and platelet count among the groups (Fig 2). ICC of late phase image visual assessment between two readers was 0.85.Discussion
Recently, various methods have been reported for hepatic function and fibrosis assessment that use hepatocyte phase images of Gd-EOB-DTPA. However, these methods require complicated calculations. In contrast, our study is based on the uptake rate of Gd-EOB-DTPA that allowed us to demonstrate that the visual assessment of late phase images is useful for assessing hepatic function and fibrosis. Our findings are consistent with the ones presented by Kessel et al.7; they reported that Gd-EOB-DTPA uptake in hepatocytes for normal hepatic function was significantly faster than that for abnormal hepatic function. Gd-EOB-DTPA uptake in normal hepatocytes began at 90 seconds after injection. However, abnormal hepatocyte can not uptake of Gd-EOB-DTPA, Gd-EOB-DTPA will be re-diffused into the blood vessels.6 Furthermore, in patients with liver cirrhosis, the peak during the washout phase is known to be flattened, extended, and delayed because of hepatic fibrosis.8 Similarly, the observed delay in the arrival and uptake rate of Gd-EOB-DTPA during the late phase is caused by the presence of abnormal hepatic function and hepatic fibrosis. Therefore, we conclude that these phenomena are causing differences in the SI values between hepatic parenchyma and hepatic vein in late phase images. Moreover, MRI operators may be possible that it can decided reduction of delayed time in hepatobiliary phase using this method. This judgment to shorten the examination time, improve examination throughput, and minimize patient’s suffering.7 This study has several limitations. Hepatic function and fibrosis assessment did not provide any liver biopsy data. However, LSR is one of the superior methods for hepatic functional assessment. Furthermore, FIB-4, APRI, and platelet count are known for their usefulness as non-invasive markers for hepatic fibrosis. Liver biopsy is almost replaced by non-invasive methods such as the FIB-4 and APRI nowadays.9-11 Thus, when no hepatic biopsy data is available, the selection of these markers is considered to be an appropriate procedure.Conclusion
In conclusion, hepatic function and fibrosis might be assessed by visual assessment of late phase images in Gd-EOB-DTPA enhanced MRI.Acknowledgements
My heartfelt appreciation goes to Kazuya Katayama whose comments and suggestions were of inestimable value for my study. I would also like to express my gratitude to my family for their moral support and warm encouragement.References
1. Taro Y, Azusa K, Osamu M, et al. Gd-EOB-DTPA-enhanced Magnetic Resonance Imaging and Alpha-fetoprotein Predict Prognosis of Early-Stage Hepatocellular Carcinoma. Hepatology. 2014 November; 60(5): 1674–1685.
2. Utaroh M, Tomoaki I, Hironobu S, et al. Liver Parenchymal Enhancement of Hepatocyte- Phase Images in Gd-EOB-DTPA-Enhanced MR Imaging: Which Biological Markers of the Liver Function Affect the Enhancement? JOURNAL OF MAGNETIC RESONANCE IMAGING. 2009; 30: 1042–1046.
3. Akihiro N, Yasuhiro U, Tsuyoshi T, et al. Quantitative analysis of liver function using superparamagnetic iron oxide- and Gd-EOB-DTPA-enhanced MRI: Comparison with Technetium-99m galactosylse- rum albumin scintigraphy. European Journal of Radiology. 2012 Jun; 81(6): 1100-1104.
4. Motosugi U, Ichikawa T, Oguri M, et al. Staging liver fibrosis by using liver-enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magnetic resonance imaging. 2011 Oct; 29(8): 1047–1052.
5. Watanabe H, Kanematsu M, Goshima S, et al. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging - preliminary observations. Radiology. 2011 Apr; 259(1): 142–150.
6. Lee NK, Kim S, Lee JW, et al. Biliary MR Imaging with Gd-EOB-DTPA and Its Clinical Applications. Radiographics. 2009 Oct; 29(6): 1707-1724.
7. C. S. van Kessel, W. B. Veldhuis, M. A. A. J. van den Bosch, et al. MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterization. Eur Radiol 2012; 22: 2153–2160.
8. Drop A, Rosińska-Bogusiewicz K, Zbańska- Klonowska K, et al. Dynamic CT of hepatic cirrhosis. Ann Univ Mariae Curie Sklodowska 2002; 57(2):39–46.
9. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; Jul; 46(1): 32-36.
10. Li Q, Ren X, Lu C, et al. Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN: A retrospective cohort study. Medicine(Baltimore) 2007 Mar; 96(12): e6336.
11. Afdhal NH, Bacon BR, Patel K, et al. Accuracy of Fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C:a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13: 772–779.
Figures
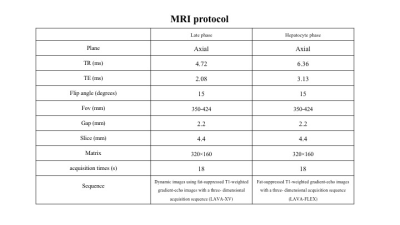
Table 1. MRI protocol
Note: The late and hepatocyte phase images were obtained at 90 seconds and 20 minutes after Gd-EOB-DTPA injection. All patients were performed intravenous administration of 0.1ml per kilogram body weight of Gd-EOB-DTPA at a rate of 2 ml/s through a 22-gauge intravenous catheter using a power injector.
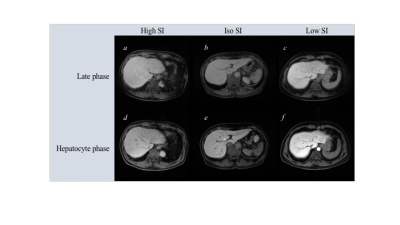
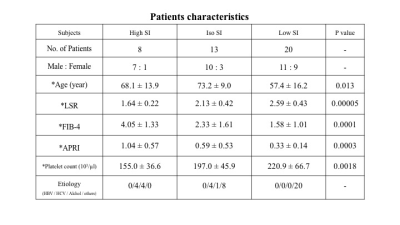
Table 2. Patients characteristics
Note: *Data are mean ± standard deviation.
P value was obtained with the Kruskal-Wallis t test.
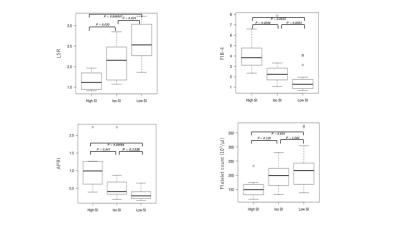
Figure 2. Box and whisker plot of LSR, FIB-4, APRI, and platelet count in three groups.