2481
Association between visceral adipose tissue and hepatic steatosis and steatohepatitis in obese patients1Radiology, Mayo Clinic, Rochester, MN, United States, 2Radiology, Hacetep University, Ankara, Turkey, 3Radiology, Tan Tock Seng Hospital, Singapore, Singapore, 4Pathology, Mayo Clinic, Rochester, MN, United States, 5Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States
Synopsis
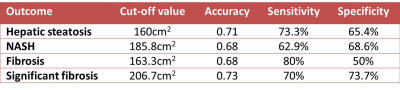
In this study we measured visceral adipose tissue (VAT), proton density fat fraction (PDFF) of liver, liver volume and correlated with liver biopsy features in obese patients at risk for non-alcoholic fatty liver disease (NAFLD). VAT shows moderately significant association with PDFF and liver volume. VAT also shows weak but significant correlation with hepatic steatosis, non-alcoholic steatohepatitis (NASH) and fibrosis at histology. Liver volume was significantly larger in patients in hepatic steatosis, NASH and fibrosis. VAT has moderate accuracy for detection of hepatic steatosis (0.71), NASH (0.68), fibrosis (0.68) and significant fibrosis (0.73) at histology.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in USA. Nearly 25% of obese patients have NAFLD. About 20% patients with NAFLD will develop nonalcoholic steatohepatitis (NASH) that can lead to fibrosis and eventually cirrhosis if untreated.1 The current method for diagnosis of NASH is liver biopsy. Although body mass index (BMI) is an independent predictor of NAFLD, body fat composition particularly visceral adipose tissue (VAT) is known have association with hepatic steatosis even in non-obese individuals. The ability of VAT quantification on MRI to predict NASH not well established.Purpose
To determine the association between visceral adipose tissue (VAT) and proton density fat fraction (PDFF) of the liver and with hepatic steatosis and non-alcoholic steatohepatitis score at histology in an obese patient s undergoing bariatric surgery.Methods
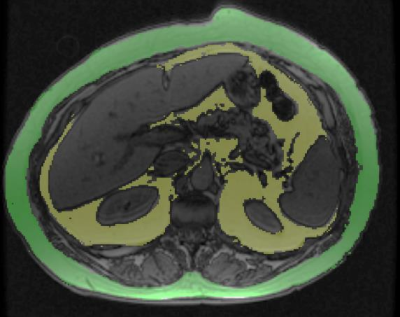
In this institutional review board approved study, 87 obese patients (defined as BMI≥30 kg/m2) scheduled to undergo bariatric surgery for weight-loss; had an MRI within 3 months of surgery and an intraoperative liver biopsy. A non-contrast enhanced MRI was performed on a whole-body GE signa HDxt 1.5T scanner. IDEAL-Q for PDFF quantification, in- and opposed phase sequences were also obtained as part of the MRI protocol. All MRI sequences included the entire liver. VAT area was quantified at L2-L3 level on opposed phase images using a semi-quantitative software (RIL contour) using signal intensity based painting of the visceral fat by one reader (fig.1). Subcutaneous adipose tissue (SAT) quantification was not possible in most patients as the abdominal wall touched the magnetic bore and SAT could not be accurately quantified. Another reader calculated liver volume (Lvol) and spleen volume (Svol) using a semiautomated 3D volume tool available on PACS (Visage version 7.1.13). A third reader calculated PDFF of liver by drawing four regions of interest on both lobes of liver on fat fraction map and a mean PDFF was obtained and expressed as percentage (%). An expert hepatopathologist reviewed the liver biopsy and graded the hepatic steatosis (HS), lobular inflammation, hepatocyte ballooning for NAS score and fibrosis stage (F-stage). Spearman’s correlation was performed between VAT and BMI, PDFF, Lvol, Svol, HS grade, NAS score. Correlation coefficient (ρ) > 0.7 was considered strong correlation, ρ=04 to 0.7 were considered as moderate correlation and ρ<0.4 was considered weak correlation.Results
The median and range of quantitative variables are summarized in Table.1. The median BMI, Lvol, Svol, PDFF and VAT were 44.6kg/m2 2504cc, 322.7cc, 11.1% and 176.4cm2 respectively. VAT showed moderate correlation with Lvol (ρ=0.51, p<0.0001) and PDFF (ρ=0.43, p<0.0001). There was no significant correlation between VAT and Svol (ρ=0.19, p=0.08), and with BMI (ρ=0.16, p=0.14). At histology, 61 patients had hepatic steatosis and 26 patients no hepatic steatosis. The median NAS score of the study population was 2 (range 0 to6). NASH was present in 36 patients and not present in 51 patients. 30 patients had fibrosis (F1=21; F2=7; F3=2 and F4=1) and 56 patients had no fibrosis (F0). There was a weak but significant correlation of VAT with HS grade (ρ=0.34, p<0.01) and NAS score (ρ=0.28, p<0.01) and fibrosis stage (ρ=0.25, p=0.02). VAT was significantly larger in patients with hepatic steatosis than those without (187cm2 vs. 136cm2, p<0.01); in patients with NASH than those without (197cm2 vs. 162cm2, p<0.01) and in patients with hepatic fibrosis than those without (169cm2 vs.203cm2, p<0.01). Receiver operating curve analysis showed that VAT ≥160cm2 had 0.71 accuracy for predicting HS; VAT≥186cm2 had 0.68 accuracy for predicting NASH and VAT≥163cm2 had 0.68 accuracy for predicting fibrosis and VAT≥206.7cm2 has 0.73 accuracy for significant fibrosis. Lvol was also larger in patients with NASH (3041cc vs. 2401cc, p<0.01) and in those with fibrosis (3062cc vs.2383cc, p<0.01).Discussion
VAT is moderately but significantly associated with hepatic steatosis, NASH and fibrosis in obese patients. Our findings confirm the previous reports that reported such association(1). Our study is probably the largest series with MRI derived VAT and correlating with histologically confirmed NAFLD. There was no significant correlation between VAT and BMI which is consistent with literature as opposed to the SAT. Our study results suggest that patients with larger VAT are at increased risk of hepatic steatosis, NASH and hepatic fibrosis. VAT may therefore be a useful parameter for non-invasive prediction of NASH. More studies in this direction are required for confirmation of our study results and explore VAT for its utility in follow up assessment of NAFLD patients following treatment. Liver volume was also moderately associated with VAT suggesting a significant association. Liver volumes tended to be larger in patients with NASH and in those with fibrosis suggesting a possible role as a biomarker. However in our study population there were only 3 patients with advanced fibrosis. Future studies that include patients with mild as well as advanced fibrosis would be useful to determine the utility of VAT in predicting advanced fibrosis.Conclusion
VAT has moderate accuracy in predicting presence of hepatic steatosis, NASH and significant fibrosis.Summary
Visceral adipose tissue is a potential non-invasive biomarker for prediction of hepatic steatosis, non-alcoholic steatohepatitis and hepatic fibrosis in patients at risk of non-alcoholic fatty liver disease.Acknowledgements
No acknowledgement found.References
1: Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, Cho EJ, Lee JH, Kim HY, Kim YJ, Kim CY. Visceral Obesity Predicts Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Medicine (Baltimore). 2015 Dec;94(48):e2159. doi: 10.1097/MD.0000000000002159. PubMed PMID: 26632897; PubMed Central PMCID: PMC4674200.
2: du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, Van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 Sep;149(3):635-48.e14. doi: 10.1053/j.gastro.2015.05.044. Epub 2015 May 28. PubMed PMID: 26028579.
Figures