2480
Limits of Fat Quantification with Chemical Shift Encoded Magnetic Resonance Imaging in the Presence of Iron Overload1Radiology, University of Wisconsin - Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 4Medicine, University of Wisconsin - Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Quantitative confounder-corrected chemical shift encoded MRI estimates of proton density fat fraction (PDFF) are corrected for R2* signal decay. However, at very high levels of iron overload, the observed signal in CSE-MRI decays rapidly (high R2*), complicating the separation of water and fat signals, and therefore the quantification of PDFF. This work characterized the degree of iron overload above which the quantification of PDFF is limited and developed practical clinical and research guidelines for PDFF estimation protocol design for 1.5T and 3.0T.
Introduction:
Intracellular accumulation of triglycerides within hepatocytes is the earliest and hallmark feature of nonalcoholic fatty liver disease (NAFLD). Iron overload is also common with chronic liver disease and often coexists with hepatic steatosis in patients with NAFLD1. Quantitative chemical shift encoded MRI (CSE-MRI) measures liver fat based on estimates of the proton density fat fraction (PDFF) corrected for all relevant confounding factors, including R2* signal decay. However, at very high levels of iron overload, the observed signal in CSE-MRI decays rapidly (high R2*), complicating the separation of water and fat signals, and therefore the quantification of PDFF2. Therefore, the purpose of this work is to characterize the degree of iron overload above which the quantification of PDFF is limited and to develop practical clinical and research guidelines for PDFF estimation protocol design for 1.5T and 3.0T.Theory:
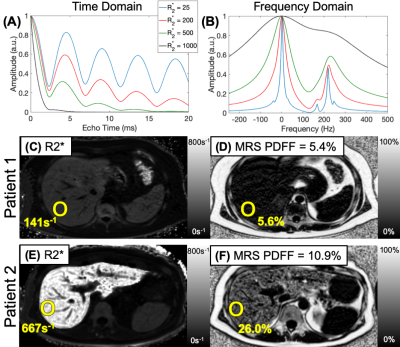
As shown in Figure 1, dramatic differences in the CSE signal evolution and spectroscopy experiments can be observed depending on the R2* signal decay. At severely elevated R2* levels the water and fat peaks coalesce, severely limiting the ability of CSE-MRI to estimate PDFF as seen in the representative maps in Figure 1 C-F.Methods:
Cramér–Rao lower bound (CRLB) SimulationsCRLB analysis for different field strengths, fat fractions, and echo time combinations were used to identify R2* values above which PDFF estimation becomes unreliable. This R2* threshold is based on an effective number of signal averages (NSA) that produces a root mean square error (RMSE) equal to the coefficient of repeatability of 4.7% from a recent meta-analysis3.
Monte Carlo Simulations
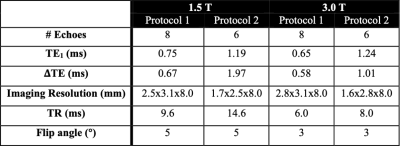
Monte Carlo simulations with PDFF values of 0,10, and 20%, and R2* values ranging from 0-1000s-1 were compared to the CRLB. Two commonly used clinically feasible acquisition protocols were investigated, one with an early first echo and short echo spacing, and another with a later first echo and longer echo spacing (Table 1).
In Vivo Experiments
A prospective study was performed in patients with known or suspected iron overload after obtaining approval from our local Institutional Review Board (IRB), and informed written consent. Subjects were imaged on a clinical 1.5T MRI system (HDxt, GE Healthcare, Waukesha, WI) using an 8-channel torso coil (USA Instruments, Cleveland, OH) and a clinical 3.0T MRI system (Discovery MR750, GE Healthcare, Waukesha, WI) using a 32-channel torso coil (NeoCoil, Pewaukee, WI). The two acquisition protocols (Table 1) were used with a quantitative complex confounder-corrected CSE-MRI method4. Further, a Single Voxel Multi-Echo Stimulated Echo Acquisition Mode (STEAM) MR spectroscopy (MRS) method5 was used as the reference standard for PDFF.
CSE-MRI acquisitions were reconstructed offline using a non-linear maximum likelihood estimator algorithm4. MRS acquisitions were analyzed through a fully automated processing of the multi-echo STEAM sequence6. Reconstructed PDFF and R2* maps were analyzed by placing a single region of interest (ROI) in the right lobe of the liver. The absolute error from each PDFF map was calculated as the difference between the average value in the ROI and the MRS estimate.
Results:
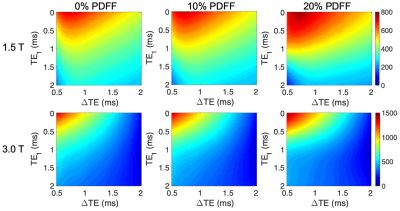
Cramér–Rao lower bound (CRLB) SimulationsThe minimum variance of the CRLB predicts that an NSA of approximately 0.2 produces a RMSE of the pixel by pixel estimate below the coefficient of repeatability from Yokoo et al3. Figure 2 summarizes the CRLB analysis, where the maximum R2* for each $$$TE_1$$$ and $$$\Delta{TE}$$$ pair that leads to an NSA above 0.2 is shown for a range of clinically relevant protocols.
Based off these CRLB simulations, expressions for the maximum R2* for a given $$$TE_1$$$, $$$\Delta{TE}$$$ pair where PDFF can be reliably reconstructed for any fat fraction were determined by fitting a second order polynomial to all echo time pairs and across fat fraction from 0-30%.
$$$1.5T: Max R2^*(TE_1,\Delta{TE})=740.5–345.1TE_1+28\Delta{TE}+20.7TE_1^2+94TE_1\Delta{TE}-84.1\Delta{TE}^2$$$
$$$3.0T: Max R2^*(TE_1,\Delta{TE})=1397–808.8TE_1–382.4\Delta{TE}+132.2TE_1^2+252.6TE1\Delta{TE}-89.1\Delta{TE}^2$$$
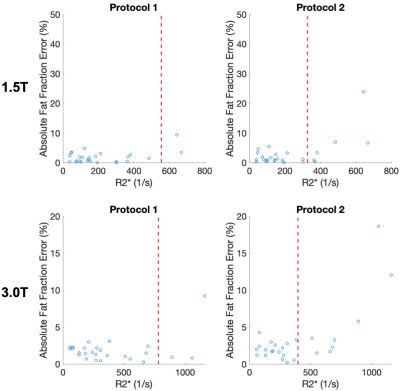
For example, the two protocols used at 1.5 T would have a maximum R2* of 522s-1 and 308s-1 (Protocol 1 and Protocol 2, respectively), above which PDFF estimates are not reliable. At 3.0T the two protocols would have a max R2* of 771s-1 and 435s-1 (Protocol 1 and Protocol 2, respectively).
Monte Carlo Simulations
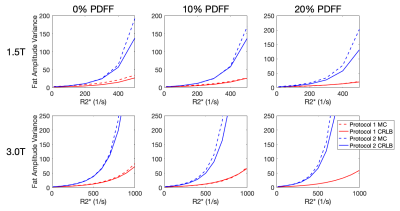
The agreement between CLRB and Monte Carlo simulations is shown in Figure 3. The choice of echo times leads to a large difference in the variance of PDFF estimates as a function of R2*.
In Vivo Experiments
A total of 26 patients were successfully recruited, with average age of 42 (11-76 range) and 17:9 men:women. There was a wide range of iron overload, with average R2* of 382s-1 (39-640s-1 range) at 1.5T and many of these patients had hepatic steatosis with average PDFF of 10% (0.8-29% range).
Figure 4 plots the absolute error in PDFF as a function of R2* for the different protocols. The acquisition with a shorter first echo and shorter echo spacing has lower PDFF errors as a function of R2*.
Discussion & Conclusions
In this work we have investigated and identified R2* thresholds above which PDFF estimation is unreliable. Practical equations were developed to identify these thresholds, based on acquisition parameters at both 1.5T and 3T. These results may provide practical clinical and research guidelines for PDFF estimation in patients who have concomitant iron overload.Acknowledgements
The authors wish to acknowledge support from the NIH R01 DK100651, K24 DK102595, R0 DK088925, R41-EB025729, R01DK117354), the Wisconsin Alumni Research Foundation (WARF) Accelerator Program, as well as GE Healthcare who provides research support to UW-Madison. Further, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Britton, L. J., Subramaniam, V. N. & Crawford, D. H. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 22, 8112–8122 (2016).
2. Hernando, D., Sharma, S. D. & Reeder, S. B. Limits of liver fat quantification in the presence of severe iron overload. in Proceedings of the 22rd Annual Meeting of ISMRM (2014).
3. Yokoo, T. et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 286, 486–498 (2017).
4. Hernando, D., Kellman, P., Haldar, J. P. & Liang, Z. P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med 63, 79–90 (2010).
5. Hamilton, G. et al. In vivo breath-hold (1) H MRS simultaneous estimation of liver proton density fat fraction, and T1 and T2 of water and fat, with a multi-TR, multi-TE sequence. J Magn Reson Imaging 42, 1538–43 (2015).
6. Hernando, D., Artz, N. S., Hamilton, G., Roldan, A. & Reeder, S. B. Fully automated processing of multi-echo spectroscopy data for liver fat quantification. in Proceedings of the 22nd Annual Meeting of the International Society of Magnetic Resonance in Medicine (2014).
Figures