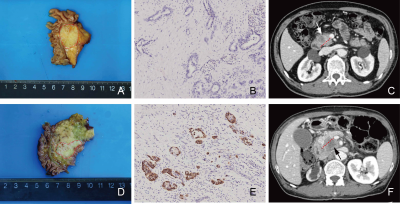
2477
Clinical Outcome of Uncinate Process Cancer versus Non-Uncinate Process Pancreatic Head Cancer: A Singe Center Retrospective Study1Radiology, Changhai hospital, Shanghai, China, 2Pancreatic Surgery, Changhai hospital, Shanghai, China, 3Pathology, Changhai hospital, Shanghai, China
Synopsis
The uncinate process has unique anatomical characteristics, which may make cancers that develop there radiopathologically different from cancers that develope elsewhere in the pancreas. Previous studies on uncinate and non-uncinate process pancreatic head cancer have yielded conflicting results. Some investigators concluded that location of pancreatic head cancer did not correlate with prognosis. Meanwhile, other investigators demonstrated a significant association between tumor location and prognosis, showing that uncinate process pancreatic head cancer was associated with a higher mortality risk versus non-uncinate process pancreatic head cancer. However, it still remains unelucidated whether tumor location is an independent prognostic factor of overall survival (OS) of pancreatic head cancer patients. In addition, it is unclear whether tumor location could guide prognostic stratification of pancreatic head cancer patients. So, the primary objectives of our study were to investigate the relationship between uncinate and non-uncinate process pancreatic head cancer and overall survival (OS) and further explore prognostic stratification between the two tumor locations and OS in different subgroups of patients.
Methods We enrolled 150 patients with pathologically confirmed pancreatic cancer who underwent multislice computed tomography from October 2014 to December 2017. All patients were divided into the uncinate process cancer group and the non-uncinate process pancreatic head cancer group, and their all characteristics were retrospectively analyzed. Multivariable Cox proportional hazards models were used to analyze association with OS.
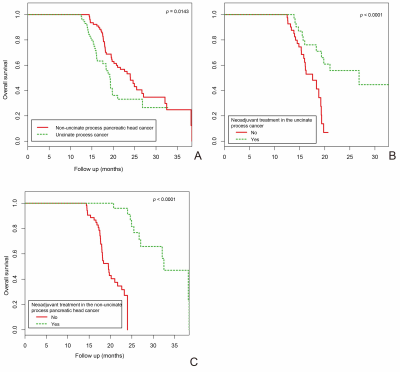
Results Patients with uncinate process cancer and non-uncinate process pancreatic head cancer accounted for 48% (n=72) and 52% (n=78) of the study cohort, respectively. OS differed significantly between the uncinate process cancer group and the non-uncinate process pancreatic head cancer group (p = 0.0143). In the two tumor locations, patients with neoadjuvant treatment had significantly longer OS than patients without neoadjuvant treatment (p < 0.0001), respectively. Lymphovascular space invasion (LVSI) played an interactive role in the association between tumor location and OS (p for interaction = 0.0002). Multivariate analyses revealed that patients with uncinate process cancer had a 2.75-fold (95% CI: 1.52-4.98) mortality risk compared to those with non-uncinate process pancreatic head cancer (p = 0.0008).
Conclusion Tumor location is an independent prognostic factor for an OS in pancreatic head cancer patients. Preoperative neoadjuvant treatment can significantly prolong OS. Keywords Pancreatic cancer; Pancreas agenesis, dorsal; Survival; Prognosis
Acknowledgements
Scientists of China (81871352), National Science Foundation for Young Scientists of China (81701689, 81601468), the 63-class General Financial Grant from the China Postdoctoral Science Foundation (2018M633714), Key Junior College of National Clinical of China, Shanghai Technology Innovation Project 2017 on Clinical Medicine (17411952200), Project of Precision Medical Transformation Application of NMMU (2017JZ42),and by Top Project of the Military Medical Science and Technology Youth Training Program (17QNP017).References
1. Kamisawa T, Okamoto A. Pancreatographic investigation of pancreatic duct system and pancreaticobiliary malformation. J Anat. Feb 2008;212(2):125-134.
2. Kuruma S, Kamisawa T, Hara S, et al. Pancreatic duct system with 2 points of embryological fusion between the ventral and dorsal pancreatic ducts. Pancreas. Apr 2013;42(3):551-552.
3. Adda G, Hannoun L, Loygue J. Development of the human pancreas: variations and pathology. A tentative classification. Anat Clin. 1984;5(4):275-283.
4. Suda K, Nobukawa B, Takase M, Hayashi T. Pancreatic segmentation on an embryological and anatomical basis. J Hepatobiliary Pancreat Surg. 2006;13(2):146-148.
5. Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg. Apr 1974;179(4):412-414.
6. Liu C, Tian X, Xie X, Gao H, Zhuang Y, Yang Y. Comparison of Uncinate Process Cancer and Non-Uncinate Process Pancreatic Head Cancer. J Cancer. 2016;7(10):1242-1249.
7. El Nakeeb A, Roshdy S, Ask W, et al. Comparative study between uncinate process carcinoma and pancreatic head carcinoma after pancreaticodudenectomy (clincopathological features and surgical outcomes). Hepatogastroenterology. Sep 2014;61(134):1748-1755.
8. Okamura Y, Fujii T, Kanzaki A, et al. Clinicopathologic assessment of pancreatic ductal carcinoma located at the head of the pancreas, in relation to embryonic development. Pancreas. May 2012;41(4):582-588.
9. Makino I, Kitagawa H, Ohta T, et al. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas. Nov 2008;37(4):358-365.
10. Ye C, Xi PC, Hu XG. Clinical analysis of uncinate process carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. Nov 2003;2(4):605-608.
11. Kang CM, Choi JY, Seong JS, et al. Pancreatoduodenectomy following neoadjuvant chemoradiation therapy in uncinate process pancreatic cancer. Pancreas. Apr 2012;41(3):467-473.
12. Suda K, Mogaki M, Matsumoto Y. Gross dissection and immunohistochemical studies on branch fusion type of ventral and dorsal pancreatic ducts: a case report. Surg Radiol Anat. 1991;13(4):333-337.
13. Malaisse-Lagae F, Stefan Y, Cox J, Perrelet A, Orci L. Identification of a lobe in the adult human pancreas rich in pancreatic polypeptide. Diabetologia. Dec 1979;17(6):361-365.
14. Kitagawa H, Ohta T, Makino I, et al. Carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Front Biosci. Jan 1 2008;13:2728-2735.
15. Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan to kagaku ryoho. Cancer & chemotherapy. Dec 2009;36(13):2495-2501.
16. Verbeke FCCS. Pathology of the Pancreas: A Practical Approach. 2013.
17. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging manual. 8 ed. New York: Springer; 2017.
18. Yamaguchi K. Carcinoma of the uncinate process of the pancreas with a peculiar clinical manifestation. Am J Gastroenterol. Aug 1992;87(8):1046-1050.
19. Suzuki T, Kuratsuka H, Uchida K, Matsumoto Y, Honjo I. Carcinoma of the pancreas arising in the region of the uncinate process. Cancer. Sep 1972;30(3):796-800.
20. Li S, Pei YQ, Du FT, et al. Surgical treatment for uncinate process carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. Nov 2002;1(4):592-594.
21. Birk D, Schoenberg MH, Gansauge F, Formentini A, Fortnagel G, Beger HG. Carcinoma of the head of the pancreas arising from the uncinate process. Br J Surg. Apr 1998;85(4):498-501.
22. Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. Apr 2015;22(4):1153-1159.
23. Gadducci A, Cavazzana A, Cosio S, et al. Lymph-vascular space involvement and outer one-third myometrial invasion are strong predictors of distant haematogeneous failures in patients with stage I-II endometrioid-type endometrial cancer. Anticancer Res. May 2009;29(5):1715-1720.
24. Dekker TJ, van de Velde CJ, van Bruggen D, et al. Quantitative assessment of lymph vascular space invasion (LVSI) provides important prognostic information in node-negative breast cancer. Ann Oncol. Dec 2013;24(12):2994-2998.
25. Briet JM, Hollema H, Reesink N, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. Mar 2005;96(3):799-804.
26. Chang ST, Jeffrey RB, Patel BN, et al. Preoperative Multidetector CT Diagnosis of Extrapancreatic Perineural or Duodenal Invasion Is Associated with Reduced Postoperative Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: Preliminary Experience and Implications for Patient Care. Radiology. Dec 2016;281(3):816-825.
27. Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. Mar 1 2019;17(3):202-210.
Figures