2473
Hepatobiliary Contrast Uptake Patterns in Liver Metastases from Pancreatic Ductal Adenocarcinoma: Prediction for Patients’ Overall Survival1Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Radiology, Gifu University, Gifu, Japan
Synopsis
Gadoxetic acid is a liver-specific contrast agent and has been reported to be useful for the detection of liver metastases in patients with pancreatic ductal adenocarcinoma (PDAC). Recently, aberrant OATP expression has been reported in liver metastases from PDAC and is associated with poor prognosis. Our results show that patients with liver metastases demonstrating heterogeneous hypointensity on hepatobiliary phase images are associated with worse overall survival compared to those with homogeneous hypointensity.
Purpose
To evaluate the relationship between the patterns of hepatobiliary phase (HBP) contrast uptake in liver metastases on gadoxetic acid-enhanced magnetic resonance imaging (MRI) and overall survival (OS) in patients with pancreatic ductal adenocarcinoma (PDAC).Materials and Methods
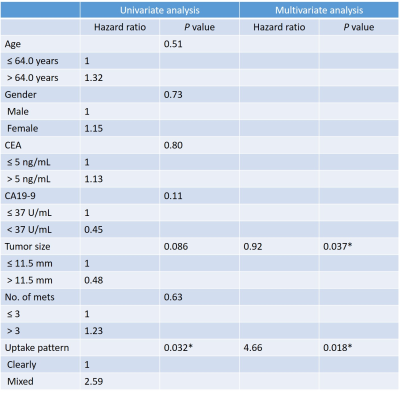
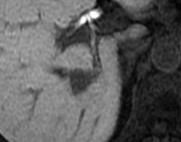
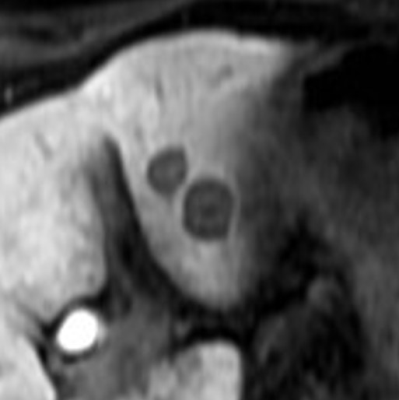
This retrospective study was approved by our institutional review board and written informed consent was waived. Fifty-seven patients (30 men and 27 women; age range, 46–92 years; mean 64.9 ± 9.2 years) with PDAC and liver metastasis who had undergone gadoxetic acid-enhanced MRI were included. A dedicated liver MRI was performed with 1.5 T MR system which included 20-min delayed three-dimensional fat-suppressed axial T1-weighted fast field-echo imaging; Qualitative and quantitative evaluation of the liver metastases was performed for signal intensity features on multiple MR sequences. Each nodule was classified as homogeneous or heterogeneous hypointense nodules. Homogeneous was defined as signal intensity similar to that of inferior vena cava. Heterogeneous nodule was defined when the signal intensity was higher than that of the inferior vena cava with areas of hyperintensity (1). During patient-by-patient analysis, those patients with both patterns of nodules were classified as belonging to the heterogeneous group. Quantitative evaluation included measurement of tumor size, number, and signal intensity. The signal intensities of liver metastasis (SInodule) and liver parenchyma (SIliver) were measured on the 20-min HBP images. Background noise was quantified as the standard deviation (SD) of the signal intensity of the liver parenchyma (SDliver). The signal-to-noise ratio (SNR) of the liver metastasis was calculated as a ratio of signal intensity of the liver metastasis to background noise. The tumor-to-liver contrast-to-noise ratio (CNR) was calculated the following equation: CNR = (SIliver – SInodule)/ SDliver. The Mann-Whitney U test was conducted to compare tumor size, SNR, and CNR between homogeneous and heterogeneous hypointense nodules. Kaplan–Meier analysis and log-rank test were conducted for univariate analysis and Cox proportional hazards regression was conducted for multivariate analysis to evaluate prognostic factors for OS in patients with PDAC and liver metastasis. A P value of less than 0.05 was considered to be significant.Results
Out of 57 patients, there were 199 liver metastases among which 61 nodules (31%) were homogeneous and 138 nodules (69%) were classified as heterogeneous hypointense nodules. Homogeneous nodules were encountered in 18 patients (32%), heterogeneous in 29 patients (51%) and both patterns co-existing in 10 patients (18%). 18 patients and 39 patients were classified into homogeneous and heterogeneous groups, respectively. Tumor size (11.7 mm vs 15.7mm; P = 0.0001) and SNR (8.9 vs 13.5; P < 0.0001) were significantly greater in heterogeneous hypointense nodules than in homogeneous hypointense nodules. The CNR was significantly lower in heterogeneous hypointense nodules than in homogeneous hypointense nodules (15.2 vs 12.8, P = 0.0016). The median follow-up duration was 9.7 (range, 1–61.3) months. Median OS was 23.6 months. Significant prognostic factors for OS in univariate analysis was contrast uptake pattern [hazard ratio (HR): 1.00 in homogeneous group, 2.59 in heterogeneous group; P = 0.032]. Mean tumor size (P = 0.037) and contrast uptake pattern (P = 0.018) were revealed statistically significant in the multivariate analysis (Table). The heterogeneous group demonstrated worse OS compared with homogeneous group (mean OS, 48.5 months vs 23.9 months; P = 0.032).Discussion
To our knowledge, there is limited data on the association of uptake of hepatobiliary contrast agent by liver metastases and patient outcome. Our study demonstrated that the patients with PDAC with metastatic nodules having heterogeneous hypointense nodules on HBP images were associated with a worse OS compared to those with homogeneous hypointense nodules. Gadoxetic acid is a liver-specific MRI contrast agent that is taken into hepatocytes by the organic anionic transporting polypeptide 1B3 (OATP1B3). Tumors of non-hepatocyte origin, including liver metastases from PDAC, do not have OATP expression and therefore do not show uptake of gadoxetic acid, therefore, are hypointense on HBP images (2). However, aberrant OATP expression has been described in liver metastases from various malignant tumors such as the colorectal, pancreas (3), gall bladder, lung, and breast. In addition, aberrant OATP1B3 expression is associated with decreased apoptosis after chemotherapy and more aggressive tumor behavior. In fact, immunohistochemically evaluated OATP1B3 expression was associated with HBP enhancement, and mixed hypointensity nodules demonstrated a worse OS in patients with liver metastasis from colorectal cancer (1). In conclusion, hepatobiliary contrast uptake pattern in liver metastasis on HBP images has the potential usefulness to predict OS in patients with PDAC and liver metastasis.Acknowledgements
Avinash R. Kambadakone has the following disclosures which are not relevant to this work: research grant support from Philips and GE Healthcare. Other authors have no relevant disclosure.References
1. Park SH, Kim H, Kim EK, Kim H, Choi DK, Chung YE, et al. Aberrant expression of OATP1B3 in colorectal cancer liver metastases and its clinical implication on gadoxetic acid-enhanced MRI. Oncotarget. 2017;8(41):71012-23.
2. Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol. 2010;195(1):13-28.
3. Kounnis V, Chondrogiannis G, Mantzaris MD, Tzakos AG, Fokas D, Papanikolaou NA, et al. Microcystin LR Shows Cytotoxic Activity Against Pancreatic Cancer Cells Expressing the Membrane OATP1B1 and OATP1B3 Transporters. Anticancer Res. 2015;35(11):5857-65.
Figures