2466
In-vivo 19F-isoflurane MR imaging in adipose tissue1Monash Biomedical Imaging, Monash University, Clayton, Australia
Synopsis
The detection of adipose tissue (AT) is of great importance in studying obesity. Isoflurane is a common fluorinated anesthetic with an excellent safety record in preclinical studies, which has a high blood–gas partition coefficient in adipose tissue. This abstract demonstrates that 19F-isoflurane can be used as an MR contrast agent for noninvasive imaging of AT in a mouse body.
Introduction
Imaging adipose tissue (AT) and defining its regional distribution is important in the study of numerous diseases associated with obesity. MRI can noninvasively image AT using 1H imaging, however, the resonance frequency of 1H in AT is very close to that of water. Brown adipose tissue (BAT) can be detected by MRI with hyperpolarized 129Xe1; however the MRI signal of 129Xe in its dissolved-phase is quite low and it depolarizes rapidly during imaging. Isoflurane contains the stable 19F fluorine isotope which can be imaged in MRI systems2. The blood–gas partition coefficient of isoflurane in AT is over 10-15 fold higher than that in brain and muscles3, providing a high concentration of 19F within AT relative to many other tissue types. In this abstract, we aimed to use isoflurane as both an anesthetic and as 19F contrast agent to image the distribution of AT in a live mouse.Materials and Methods
All MRI experiments were performed on a Bruker BioSpec 9.4T scanner (Bruker BioSpin GmbH, Ettlingen, Germany) with a 1H/19F dual-channel 40mm volume coil. A gas phantom with 5% isoflurane and a water phantom dissolved with isoflurane were used to measure the T2 relaxivities of gas and dissolved-phase isoflurane. One C57Bl6 mouse was anesthetized with 1.5% isoflurane in oxygen for 45 mins before imaging and anaesthesia maintained on 1-1.5% isoflurane during all the 19F imaging scans.Data Acquisition
The frequency of gas-phase and dissolved-phase 19F-isoflurane was set at 376.1968 MHz (Chemical shift offset = -83.205 ppm) and 376.1667 MHz (Chemical shift offset = -80.75 ppm), respectively. A 3D multi slice multi echo (MSME) sequence was applied to image the 19F isotope within isoflurane. The MRI parameters for imaging the gas phantom were: TR=1000 ms, TE=2.77 ms, echo spacing=2.77 ms, echo averages=1, echo images=8, matrix=32*32*8, FOV=32*32*240 mm2, and averages=40. The MRI parameters for imaging the dissolved-phase 19F phantom were: TR=3000 ms, TE=11 ms, echo spacing=2 ms, echo averages=10, echo images=50, matrix=32*32*8, and FOV=32*32*240 mm3. The parameters for in-vivo 19F imaging were: TR=2000ms, TE=328 ms, echo spacing=3.2ms, echo averages = 204, echo images = 1, matrix = 40*40*8, scan time = 10 mins 40 s, axial plane and sagittal with FOV=32*32*24 mm3, and coronal plane with FOV=32*32*56mm3.Results and Discussion
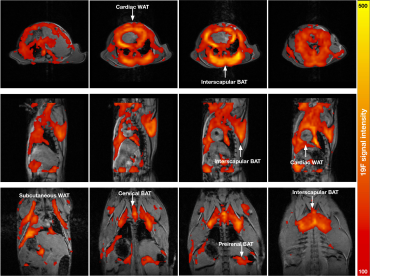
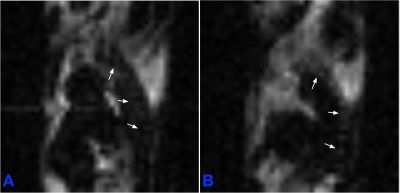
The T2 relaxivity measured in the gas- and dissolved-phase isoflurane were 7.9±0.1 ms and 1583.1±29.3 ms, respectively. Figure 1 shows the concentration of isoflurane in a mouse body in axial (Figure 1 first row), sagittal (Figure 1 second row) and coronal (Figure 1 third row) planes. In this study, the mouse inhaled isoflurane for 45 mins before imaging with a long-TE MSME sequence. The gas-phase 19F signal cannot be seen in the mouse lung because of the short T2 relaxivity of isoflurane gas (See Figure 1). The 19F signals in muscles, liver and kidneys were not visible, possibly because of the small blood–gas partition coefficients in these regions3. We scanned the mouse after it inhaled isoflurane for 45 mins which provided enough time for the accumulation of isoflurane in AT4. The 19F signals of isoflurane were mostly accumulated in cardiac white AT (WAT), interscapular BAT, cervical BAT, subcutaneous WAT and perirenal BAT (See Figure 1). The distribution of isoflurane in AT is quite similar with that of 18F-FDG PET5. It is interesting that the 19F signals were visible in spine (See arrows in Figure 2A) and ribs (See arrows in Figure 2B).Conclusions
19F-isoflurane has a long T2 relaxation time and a high blood–gas partition coefficient in AT, which can be used as an inhalational contrast agent for imaging AT.Acknowledgements
We would like to acknowledge the support of the Australian National Imaging Facility Fellowship.References
[1] Branca RT, He T, Zhang L, Floyd CS, Freeman M, White C, Burant A. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc Nat Acad Sci U S A. 2014 Dec 16;111(50):18001-6.
[2] Venkatasubramanian PN, Shen YJ, Wyrwicz AM. Characterization of the cerebral distribution of general anesthetics in vivo by two-dimensional 19F chemical shift imaging. Magn Reson Med. 1996;35(4):626-30.
[3] Morgan GE. Clinical Anesthesiology (5th). 2013;156.
[4] Hashimoto T, Ikehira H, Fukuda H, Ueshima Y, Tateno Y. Study of biodistribution of enflurane in rats with in vivo 19F MRI. Magn Reson Imaging. 1991;9(4):577-81.
[5] Mirbolooki MR, Upadhyay SK, Constantinescu CC, Pan ML, Mukherjee J. Adrenergic pathway activation enhances brown adipose tissue metabolism: a [¹⁸F ]FDG PET/CT study in mice. Nucl Med Biol. 2014 Jan;41(1):10-6.