2465
Quantitative Renal Function Assessment of Renal embolization1Peking University, Beijing, China
Synopsis
Renal embolization is part of a multisystemic disease and has attracted enhanced attention in recent years for its increasing incidence in the elderly.It is a significant cause of renal loss in patients who suffer from valvular cardiopathy, aortic atheromatosis, and hypercoagulable states. In this study, we attempt to investigate the feasibility of compressed sensing (CS) based DCE-MRI in the assessment of AERD in animal models.
Introduction
Renal embolization is part of a multisystemic disease and has attracted enhanced attention in recent years for its increasing incidence in the elderly[1]. It is a significant cause of renal loss in patients who suffer from valvular cardiopathy, aortic adenomatosis, and hypercoagulable states[2-5]. The definitive diagnosis is made by renal biopsy[6]. However, many patients are too acutely ill to tolerate renal biopsy. In recent years, several imaging methods including radioisotope renogram, renal angiogram, and contrast-enhanced CT have been applied to aid in the diagnosis of renal diseases. However, they are invasive and or have relatively poor sensitivity.Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has been established as a reliable method for estimating single kidney glomerular filtration rate (GFR) and assessment of renal disease[7]. In this study, we attempt to investigate the feasibility of compressed sensing (CS) based DCE-MRI in the assessment of renal embolization in animal models.
Methods
The in vivo animal experiments were approved by the Hospital Ethics Committee for Animal Research. Experiments were performed on 15 male New Zealand White rabbits (weighing 2.8–3.3 kg). Animal models of unilateral embolization were first conducted. DCE-MRI data were acquired using a GE 3.0T MR scanner (Signa ExciteTM; General Electric Medical Systems, Milwaukee, WI, USA) with 8-channel TORSOPA coil. The coronal CS DCE-MRI scan was performed with the following parameters: TR = 3.2 msec / TE = 1.3 msec, flip angle = 12°, acquisition matrix = 256 × 256 × 12, NEX=1, slice thickness = 4 mm, field of view = 180mm, CS acceleration factor = 4. In dynamic contrast-enhanced experiments, five frames of non-enhanced volumes of the kidney were acquired before the bolus administration. Then, 0.1 mmol/kg bodyweight of Gd-DTPA (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) was injected, followed by a 5.0 ml saline flush. Images were acquired immediately and the total scan time is about 8 minutes for each rabbit.Results were represented in mean ± standard deviation (SD). The student t-test was performed to compare the value of GFR between normal tissue and contra-lateral embolized lesion. P-value < 0.05 was considered statistically significant.
Results
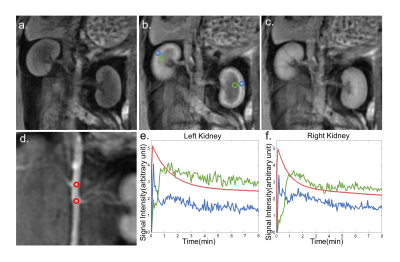
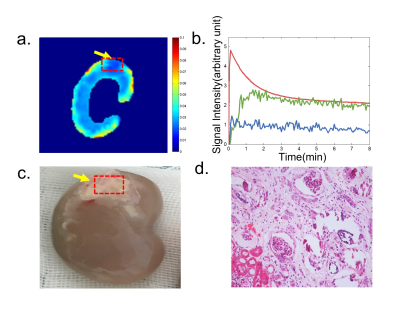
A representative CS DCE-MR image and contrast agent uptake curve in normal kidneys are shown in Fig 1. The detailed spatial resolution enables the display of the renal parenchyma and renal artery without obvious artifacts. Cortical ROIs were manually drawn (blue and green circle in Fig 1b) and slice that with a branch of the renal artery (red circle in Fig 1d) was selected for aortic ROI drawing. Representative concentration curves at a temporal resolution of 2.5s derived from corresponding ROIs are shown in Fig 1 e,f.Representative GFR map and corresponding concentration curves derived from cortical and medullary ROIs are shown in Fig 2 a,b. GFR reduction regions on the GFR map (Fig 2a) matched closely to the heavily embolized regions in the renal specimen (Fig 2c), but the lesion region in the GFR map showed a certain degree of underestimation compared with the specimen. In histological findings (Fig 2d), the glomeruli showed ischemic and wrinkled features with thickening change of Bowman’s capsule, and necrosis of the renal tubular epithelial cells was observed, the basement membrane was exposed. Meanwhile, the brush border of some tubular epithelial cells fell off and the tubular epithelial cells became flat and tubular lumen expanded.
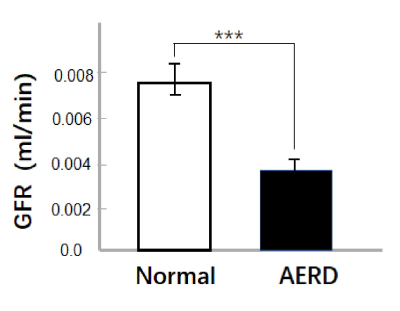
A total of 17 lesions was found in all rabbits by the renal specimen and confirmed by histological findings. 14 lesions were found by CS DCE-MRI and the lesion size is 0.14 ± 0.07 cm2. For the GFR comparison, GFR of the embolized lesion was significantly lower than GFR of normal tissue (0.0038 ± 0.005 ml/min vs 0.0075 ± 0.0008 ml/min, P<0.0001) in Fig 3.
Discussion
Once the emboli enter the blood circulation, it will stay in a small artery about 150-200 μm in diameter[9] and further causing small artery occlusion and inflammation. The focal feature of embolization, placing a greater demand on the spatial resolution of imaging modality. In this study, the spatial resolution of CS DCE-MRI is 0.7mm*0.7mm. The high resolution and reduced partial volume effect enabled precise visualization of lesion and detection of a majority of lesions (14/17).In this study, a temporal resolution of 2.5s was achieved and was adequate for renal DCE-MRI. By utilizing a well-established cortical compartment model[10, 11], the GFR values estimated normal kidneys with high reproducibility were close to literature reports[8, 10], which suggested that this method was able to provide reliable GFR measurements.
Conclusion
In conclusion, this preliminary animal study demonstrated the feasibility of using the CS DCE-MRI in quantitative renal function evaluation, with satisfactory reproducibility. The efficacy was carefully verified by histology findings. This CS DCE-MRI could potentially provide a valuable tool to identify renal embolization and monitor the success of therapeutic methods in clinical practice.Acknowledgements
No acknowledgement found.References
[1] SCOLARI F, RAVANI P. Atheroembolic renal disease [J]. The Lancet, 2010, 375(9726): 1650-60.
[2] FORT J. Renal artery embolism [J]. Renal Failure, 1997, 19(6): R7-R8.
[3] NICHOLAS G G, DEMUTH W E. Treatment of Renal-Artery Embolism [J]. Arch Surg-Chicago, 1984, 119(3): 278-81.
[4] KORZETS Z, PLOTKIN E, BERNHEIM J, et al. The clinical spectrum of acute renal infarction [J]. Isr Med Assoc J, 2002, 4(10): 781-4.
[5] KANSAL S, FELDMAN M, COOKSEY S, et al. Renal artery embolism - A case report and review [J]. J Gen Intern Med, 2008, 23(5): 644-7.
[6] WILSON D M, SALAZER T L, FARKOUH M E. Eosinophiluria in atheroembolic renal disease [J]. The American journal of medicine, 1991, 91(2): 186-9.
[7] HAHN O M, YANG C, MEDVED M, et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging Pharmacodynamic Biomarker Study of Sorafenib in Metastatic Renal Carcinoma [J]. Journal of Clinical Oncology, 2008, 26(28): 4572-8.
[8] CHEN B, ZHANG Y, SONG X, et al. Quantitative estimation of renal function with dynamic contrast-enhanced MRI using a modified two-compartment model [J]. PloS one, 2014, 9(8): e105087.
[9] ELIOT R S, EDWARDS J E, KANJUH V I. Atheromatous Embolism [J]. Circulation, 1964, 30(4): 611-&.
[10] ANNET L, HERMOYE L, PEETERS F, et al. Glomerular filtration rate: Assessment with dynamic contrast-enhanced MRI and a cortical-compartment model in the rabbit kidney [J]. Journal of Magnetic Resonance Imaging, 2004, 20(5): 843-9.
[11] BUCKLEY D L, SHURRAB A E, CHEUNG C M, et al. Measurement of single kidney function using dynamic contrast-enhanced MRI: Comparison of two models in human subjects [J]. Journal of Magnetic Resonance Imaging, 2006, 24(5): 1117-23.
Figures