2458
Multiparametric-MRI in the Evaluation of Early diabetic kidney damage1Medical Imaging Center, First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, China, Guangzhou, China, 2Medical Imaging Center, First People's Hospital of Kashgar, Xinjiang, china., Xinjiang, China, 3MR Research, GE Healthcare, Beijing, 100176, China., Beijing, China
Synopsis
Multiparametric-MRI (mp-MRI) has shown promising results in the diagnosis of clinical disease. It provides us an approach to perform investigation from multiple dimension and avoids to ignore the worthwhile diagnosis information. Previous studies indicated that the perfusion and dispersion of renal parenchyma have been changed in diabetic mellitus (DM) without biochemical indicators of significant renal damage. However, the convergence and divergence of those diffusion and perfusion related MRI parameters in the evaluation of DM associated kidney damage is still unclear. To assess the renal functional changes in DM patients, three MRI modalities were applied in current study.
Synopsis
Multiparametric-MRI (mp-MRI) has shown promising results in the diagnosis of clinical disease. It provides us an approach to perform investigation from multiple dimension and avoids to ignore the worthwhile diagnosis information. Previous studies indicated that the perfusion and dispersion of renal parenchyma have been changed in diabetic mellitus (DM) without biochemical indicators of significant renal damage. However, the convergence and divergence of those diffusion and perfusion related MRI parameters in the evaluation of DM associated kidney damage is still unclear. To assess the renal functional changes in DM patients, three MRI modalities were applied in current study.Introduction
Diabetic nephropathy (DN) is the leading cause of chronic renal disease worldwide. Early diagnosis and treatment can delay or prevent the onset of DN. There is an urgent need to discover the new noninvasive imaging methods for detecting early renal injury. Previous studies indicated that the perfusion and dispersion of renal parenchyma have been changed in diabetic mellitus (DM) without biochemical indicators of significant renal damage1. Further, the renal cortex is mainly characterized by elevated perfusion, and the medulla is associated with anisotropic diffusion. However, the convergence and divergence of those diffusion and perfusion related MRI parameters in the evaluation of DM associated kidney damage is still unclear. To assess the renal functional changes in diabetic patients without significant proteinuria, three MRI modalities, intravoxel incoherent motion (IVIM), diffusion tensor imaging (DTI), and blood oxygenation level dependent imaging (BOLD), were applied in current study.Methods
For each participant, three MRI sequences including multiple b-values DWI, DTI and BOLD were applied to acquire the imaging data on a 3.0-Tesla MR system (Discovery MR750, General Electric, Milwaukee, WI, USA). A total of thirty type II diabetes (DM group) and thirty sex- and age-matched control subjects (Control group) were enrolled in the study. For all participants, the urinary microalbumin, the serum creatinine, serum uric acid, HbAlc, and fasting blood glucose were measured to calculate Estimate the glomerular filtration rate (eGFR) and albumin–creatinine ratio (ACR) before MR scan. Additionally, the individuals in the DM groups should underwent fundus examination. All subjects fasting for 4 hours before MR scan. Regions of interest (ROIs) were placed in the location of renal cortex and medulla on ADC, D, D*, f, ADCDTI, FA and R2* maps by two experienced radiologists. The two-sample t-test were applied to test the differences of those MRI derived metrics between the two groups. Receiver operating characteristic (ROC) curve was plotted, and the area under the ROC curve (AUC) was calculated to assess the performance of each MR index in differentiating between DM and non-DM.Results and Discussion
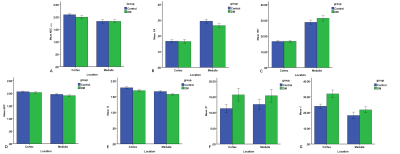
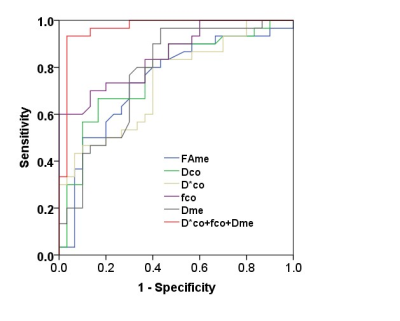
The DCo* and fCo values of renal cortex in diabetic patients were significantly higher than those in the control group (P < 0.01). The D value of the cortex and the FAMe and DMe values of the medulla of the diabetic patients were significantly lower than those of the control group (P < 0.01) (Fig.1). The areas under the curve (AUCs) in differentiating between DM and non-DM for FAMe, DCo, DMe, DCo*, and fCo were 0.75, 0.77, 0.74, 0.85, and 0.78, respectively. Combining DMe, DCo*, and fCo achieved a higher AUC of 0.97(Fig. 2). No significant differences in other parameters were found between the two groups. Parameters related to renal cortical perfusion measured by IVIM were significantly higher in DM patients (DCo* =15.68×10-3mm2/sec and fCo =31.95﹪ ) than in controls (DCo* =11.32×10-3mm2/sec and fCo =24.09﹪ ), suggesting an abnormally high microcirculation perfusion in diabetic patients prior to abnormal. This might be related to high blood sugar, high-protein diet, increased renal tubular flow and ultrafiltration volume caused by the renin–angiotensin–aldosterone system (RAAS), increased blood vessels, and the relatively broader diameter of the renal tubules2,3. Our study found that in the early stages of diabetes, the renal cortex showed elevated perfusion, but the renal medulla did not. A possible explanation is that the renal cortex has some reserve capacity for the damage, whereas the medulla is susceptible to injuries from ischemic hypoxia, toxic substance accumulation, and so on4,5,6. The renal cortical D value in the diabetic group was significantly lower than that in the control group, indicating limited water molecule diffusion in both the cortex and medulla. This might be related to a series of early histological characters, including glomerular basement membrane thickening, tubular epithelial cell swelling, mesangial expansion, accumulation of transparent material in the glomerulus basement membrane epithelium, capillary and capsular adherence, and compromised energy metabolism2,4,7-10. The current study showed a decreasing trend in FA value of the renal medulla from the control group to the DM group. Structural changes in the kidney of patients with DM might reduce the extracellular space and renal water content and consequently limit the diffusion of water molecules11. Furthermore, slight infiltration of inflammatory cell interstitium in the early course resulted in an increase in the cell density12, which also contributed to the limited diffusion.Conclusion
Our results demonstrated that IVIM and DTI has an advantage in assessing early renal damage in DN. The renal cortex is mainly characterized by elevated perfusion, and the anisotropic diffusion is reduced in the medulla. However, both of renal cortex and medulla diffusion are reduced.Acknowledgements
This study has received funding by the Guangdong Science and Technology project in China(grant no.2017A030313901) and Guangzhou Science and Technology project (grantno.201804010239). The funders had no role in study design, data collection and analysis,decision to publish, or preparation of the manuscript.References
1. Feng, Y. Z. et al. Intravoxel incoherent motion (IVIM) at 3.0?T: evaluation of early renal function changes in type 2 diabetic patients. J. Abdominal Radiology 43, 2764-2773 (2018).
2. Caramori, M. L., Fioretto, P. & Mauer, M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions.J. Diabetes. 52, 1036-1040 (2003). 3. Su, J. et al. Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms' tumor-1 protein used as a podocyte marker. J. Diabetes research and clinical practice. 87, 167-175 (2010).
4. Peng, X. G. et al. Renal lipids and oxygenation in diabetic mice: noninvasive quantification with MR imaging. J. Radiology 269, 748-757 (2013).
5. Soininen, P., Kangas, A. J., Wurtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. J. Circulation. Cardiovascular genetics. 8, 192-206 (2015).
6. Cai, X. R. et al. Assessment of renal function in patients with unilateral ureteral obstruction using whole-organ perfusion imaging with 320-detector row computed tomography. PloS one. 10, e0122454; 10.1371/ journal.pone. 0122454 (2015).
7. Welch, W. J. Intrarenal oxygen and hypertension.J. Clinical and experimental pharmacology & physiology. 33, 1002-1005 (2006).
8. Luo, B. et al. LOX-1-Targeted Iron Oxide Nanoparticles Detect Early Diabetic Nephropathy in db/db Mice.J. MIB : the official publication of the Academy of Molecular Imaging. 17, 652-660 (2015).
9. Bakris, G. L. Microalbuminuria: prognostic implications.J. Current opinion in nephrology and hypertension 5, 219-223 (1996). Soininen, P., Kangas, A. J., Wurtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. J. Circulation. Cardiovascular genetics. 8, 192-206 (2015).
10. Xiao, L. et al. Rap1 ameliorates renal tubular injury in diabetic nephropathy. J. Diabetes. 63, 1366-1380 (2014).
11. Prasad P, Li LP, Halter S, Cabray J, Ye M, Batlle D. Evaluation of renal hypoxia in diabetic mice by BOLD MRI. Investigative radiology. 2010,45,819-822.
12. Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clinical nephrology. 1998, 49,1-8.
Figures