2452
A Deuterated 13C-Urea Reference to Eliminate 1H Imaging Artifacts in Hyperpolarized 13C MRI Studies1Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2The University of Texas MD Anderson Cancer Center UT Health Graduate School of Biomedical Sciences, Houston, TX, United States, 3Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Multiparametric and hyperpolarized (HP) 13C Magnetic Resonance Imaging (MRI) are attractive multi-modality methods for prostate cancer imaging studies because of their non-invasiveness and characterization of changes in tumor metabolism. A 13C-urea calibration reference is required when conducting HP 13C MRI studies, which must be heavily doped with Gd+ to facilitate rapid calibrations. Care must be taken to ensure that the highly relaxed 1H signal does not compromise DCE-MRI via hyperintense imaging artifacts. Deuteration and lyophilization of 13C-urea can be useful in creating a calibration reference that does not lead to imaging artifacts in 1H Images.
Introduction
The ability to manage and predict outcome for patients with prostate cancer is a critically important unmet clinical need. In regards to prostate cancer imaging, accurate disease characterization is at the forefront of importance. Multi-modality information gathered by noninvasive imaging techniques1 is a promising method to achieve this goal. HP 13C MRI is an emerging metabolic imaging method that can result in a greater than 10,000-fold gain in signal intensity.2 When paired with dynamic contrast-enhanced (DCE-) MRI, these modalities can inform on the quantitative analysis of 13C signal evolution.3 Currently, 13C-enriched reference standards are required to enable fast and accurate calibration for HP 13C studies. However, care must be taken to ensure that the reference is compatible with both 1H and 13C acquisitions. The goal of this study was to optimize the reagents used in a 13C-urea reference for a dual-tuned 13C/1H endorectal coil (ERC) and minimize imaging artifacts in metabolic and multiparametric MRI studies involving hyperpolarized [1-13C]-pyruvate.Methods
Due to a high amount of Gd+ doping for the purpose of reducing the spin-lattice relaxation time (T1) of urea, the 1H signal produced by an undeuterated 13C-urea reference was rapidly relaxed, resulting in severe imaging artifacts in DCE-MRI. Hyperintense ringing artifacts in 1H images were mitigated by reducing the 1H concentration in a 13C-urea reference via deuteration and lyophilization.Several references were fabricated and their SNR was compared using 1H and 13C imaging sequences on a 3T MRI scanner (MR750, GE Healthcare, Waukesha, WI, USA). 1H prostate phantom imaging using a T1-weighted 3D FSPGR sequence (3.068 ms repetition time, 1.344 ms echo time, 220 mm2 field of view, 3.6 mm slice thickness, 1.8 mm slice spacing, and a 20-degree flip angle) was conducted to compare image quality and mean 1H SNR of a non-deuterated urea reference and a deuterated urea reference.
Results
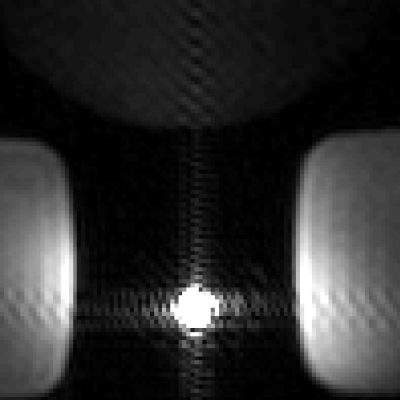
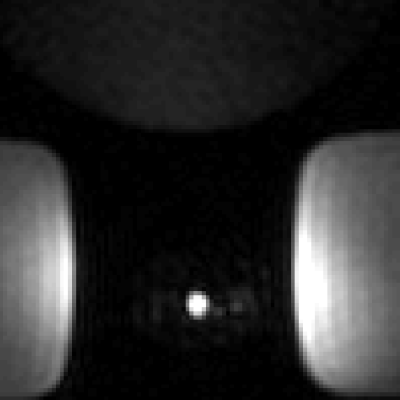
The deuterated 13C-urea reference provides strong 13C signal for calibration and an attenuated 1H signal that does not interfere with heavily T1-weighted scans. 1H 3D FSPGR MRI acquired of the ERC embedded with the non-deuterated 13C-urea reference inside a prostate phantom (Figure 1) showed hyperintense ringing artifacts in the phase and frequency encoding directions. The mean 1H SNR of the undeuterated urea reference was 2.77 x 103. 1H 3D FSPGR MRI acquired of the ERC embedded with the deuterated reference (Figure 2) showed the removal of the hyperintense ringing artifacts. The mean 1H SNR of the deuterated reference was 1.13 x 102. Therefore, the signal intensity of the non-deuterated urea reference compared to the deuterated reference showed a reduction by a factor of 25.Discussion
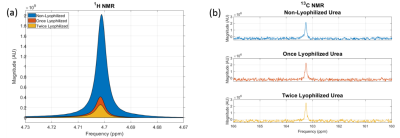
The deuterated urea reference successfully avoided the 1H imaging artifacts produced by the highly Gd+ doped, non-deuterated 13C-urea reference. The hyperintense ringing artifacts observed from the undeuterated reference would limit the diagnostic quality of the 1H images and would negatively affect forthcoming DCE-MRI scans acquired in conjunction with HP 13C MRI. The deuterated reference was able to successfully reduce the mean 1H SNR, which led to complete removal of hyperintense ringing artifacts. Furthermore, the 13C-urea signal from the deuterated reference was unaffected (Figure 3), allowing it to continue to serve as a calibration reference for future 13C studies. The deuterated reference is a fundamental 13C calibration tool which will not produce imaging artifacts during 1H MRI and thus will support the acquisition of anatomical and functional data to compliment HP 13C imaging.Conclusion
We designed a reference standard for calibration of 13C scans that does not compromise anatomic or functional 1H MRI. The deuterated urea reference showed a reduction in signal intensity when compared to a non-deuterated reference embedded in the ERC, and eliminated 1H ringing artifacts in heavily T1-weighted images. Two cycles of lyophilization of 13C-urea was sufficient to eliminate 1H ringing artifacts and can be used to improve anatomical image quality in future clinical 1H and HP [1-13C]-pyruvate MRI prostate cancer imaging studies.Acknowledgements
This work was supported by funding from the National Cancer Institute of the National Institutes of Health (R01CA211150, P30CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Kelloff GJ, Choyke P, Coffey DS, Prostate Cancer Imaging Working G. Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol. 2009;192(6):1455-1470.
2. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158-10163.
3. Bankson JA, Walker CM, Ramirez MS, et al. Kinetic Modeling and Constrained Reconstruction of Hyperpolarized [1-13C]-Pyruvate Offers Improved Metabolic Imaging of Tumors. Cancer Res. 2015;75(22):4708-4717.
Figures