2432
Using radiomics to build prediction model for diagnosing metastatic lymph node in rectal cancer basing on HR-T2 imaging
Gesheng Song1, Aiyin Li1, Jingjing Cui2, and Yan Jia2
1Shandong Provincial Qianfoshan Hospital,the First Hospital Affiliated with Shandong First Medical, Jinan, China, 2Huiying Medical Technology Co., Ltd., Beijing, China
1Shandong Provincial Qianfoshan Hospital,the First Hospital Affiliated with Shandong First Medical, Jinan, China, 2Huiying Medical Technology Co., Ltd., Beijing, China
Synopsis
MRI based radiomics machine learning model could differentiating metastatic lymph node in rectal cancer.
Introduction
Lymph node metastasis is an important risk factor for local recurrence and distant metastasis of rectal cancer [1,2]. Therefore, accurate preoperative diagnosis of lymph node metastasis in rectal cancer is of great significance to the prognosis evaluation and the formulation of individualized treatment. Recent studies have shown that magnetic resonance based radiomics analysis has important values in identifying tumor heterogeneity and can add a further dimension to the predictive power of imaging [3-5]. The purpose of this study was to investigate the the value of high resolution T2-weighted–based radiomics in diagnosing metastatic lymph node in rectal cancer.Methods
A total of 41 patients with 141 lymph nodes (72 metastatic and 69 non-metastatic) were obtained. All patients accepted high-resolution T2 examination(3.0T Discovery 750, GE Medical Systems, Milwaukee, WI). The region of interest was delineated along the contour of lymph nodes with the largest cross section. A total of 1409 features were extracted on a radiomics analysis platform (Radcloud, Huiying Medical Technology, Beijing, China) and 18 features were finally obtained after selected by ANOVA and LASSO(figure 1). A logistic regression model of machine learning was build using the 18 features and was evaluated by receiver operating characteristic (ROC), area under the ROC (AUC), sensitivity (SE) and specificity (SP). Two radiologists with different working experience in diagnosing rectal diseases diagnosed the lymph nodes respectively by HR-T2 imaging alone without knowing the pathological results. The diagnostic efficiency between radiomic predicting model and subjective diagnosis was compared. The software used was SPSS v20. P < 0.05 was considered to have statistical difference.Results
1, For the prediction model, the AUC was 0.862 in training set (SE=0.825, SP=0.873) and 0.8482 in validation set (SE=0.786, SP=0.733)(table 1,figure 2); 2, The radiomic prediction (AUC=0.760, SE=0.733 and SP=0.786) had the better results than radiologists(junior radiologist: AUC=0.557, SE=0.400 and SP=0.714; senior radiologist: AUC=0.693, SE=0.600 and SP=0.786)(table 2,figure 3).Discussion
The initial purpose was to help differentiate metastatic and non-metastatic lymph node in rectal cancer. High-resolution T2-weighted MRI-based radiomics was used on an attempt to quantify the Characteristics of metastatic and non-metastatic lymph nodes. By using the pretreatment MRI data, we developed a radiomics model with better performance for individualized, noninvasive diagnosis of metastatic lymph node in patients with rectal cancer compared with radiologists.Conclusion
By extracting the radiomic features of lymph nodes from high-resolution T2 and establishing a prediction model, it can be used to differentiate metastatic and non-metastatic lymph nodes. The diagnostic performance is better than radiologist, especially when compared with junior doctors.Acknowledgements
This study has received funding by Shandong Science and Technology Development Plan (2014SF118086), China; Shandong Medical and Health Science and Technology Development Plan (2017WS879, 2016WS0475), China.References
- Ozis SE, Soydal C, Akyol C, et al. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer[J]. World journal of surgical oncology, 2014, 12(1):1-7.
- Spinelli P, Schiavo M, Meroni E, Di Felice G, Andreola S, Gallino G, et al. Results of EUS in detecting perirectal lymph node metastases of rectal cancer: the pathologist makes the difference. Gastrointest Endosc 1999;49(6):754–8.
- Robert JG, Paul EK, Hedvig H, et al. Radiomics: image are more than pictures, they are data[J]. Radiology. 2016; 278(2):563-577.
- Wu J, Tha KK, Xing L, et al. Radiomics and radiogenomics for precision radiotherapy[J]. J Radiat Res. 2018; 59(suppl_1): i25-i31.
- Horvat N, Veeraraghavan H, Khan M, et al. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy[J]. Radiology. 2018;287(3):833-843.
Figures
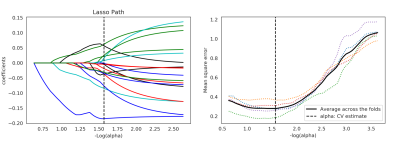
Figure 1 Lasso althorithm on feature selection. (a)Lasso path; (b)Mean square error path under different alphas. Using Lasso model, 18 features which are correspond to the optimal alpha value were selected.
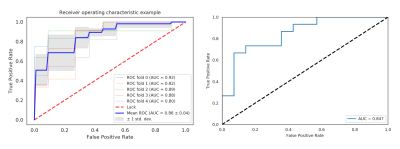
Figure 2 ROC of training and validation set. (a) training set using 5-fold cross validation and the mean AUC was 0.862; (b) validation set and the AUC was 0.848.
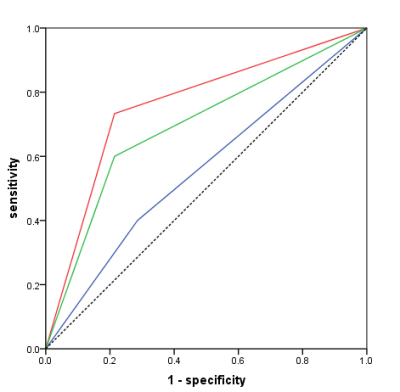
Figure 3 the ROC of radiomic (red line), reader 1(blue line) and reader 2(green line).
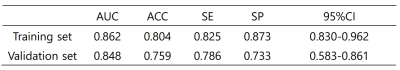
Table 1. ROC analysis by LR of training and validation set
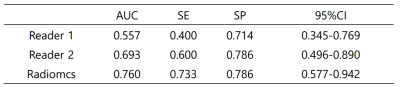
Table 2
Comparison of diagnostic efficiency between subjective diagnosis and radiomics