2425
Reduced field-of-view and multi-shot DWI acquisition techniques: prospective evaluation in prostate cancer imaging1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Diffusion weighted imaging (DWI) non-invasively evaluates tissue microstructure, relevant in the setting of prostate cancer. Distortion from susceptibility effects can confound standard single-shot echo planar imaging (ssEPI). Reduced-distortion techniques, including reduced field of view (rFOV) and multi-shot EPI (msEPI), may improve prostate imaging quality but further evaluation is warranted. Therefore, a prospective comparison of rFOV and msEPI to ssEPI, in either biopsy proven or suspected prostate cancer patients, was performed. Our results demonstrate that both rFOV and msEPI reduce distortion artifacts, and improve image quality, in comparison to ssEPI. Furthermore, ADC quantification was reproducible across these three techniques.
Introduction
Diffusion weighted imaging (DWI) non-invasively probes tissue microstructure, and is clinically useful for prostate cancer evaluation (lesion detection, characterization, biopsy targeting, and treatment planning)1-8. DWI is typically performed with single-shot echo planar imaging (ssEPI) which can be confounded by image distortion from susceptibility-related field inhomogeneity, most notably from air in the rectum.Using reduced-distortion DWI techniques, including reduced field of view (rFOV) and multi-shot EPI (msEPI), can mitigate this challenge. Recent work demonstrates that reduced-distortion DWI techniques generate reproducible quantitative diffusion measurements relative to ssEPI both in phantom experiments as well as in volunteers9-10. Additional early work evaluating rFOV also demonstrated reduced distortion in the setting of prostate cancer11-12.
However, the performance of these alternative DWI sequences, particularly msEPI, in prostate cancer patients warrants further study. Therefore, the purpose of this work is to prospectively evaluate the performance of rFOV and msEPI based DWI, in comparison to the reference standard of ssEPI, regarding image distortion and quantification of apparent diffusion coefficient (ADC) maps.
Methods
A prospective IRB-approved HIPAA compliant study was performed and informed consent was obtained. Eligible subjects who were undergoing MRI of the prostate were recruited for additional add-on research sequences.MRI acquisition/reconstruction: Imaging was performed with 3.0T MRI (750w or Premier, GE Healthcare, Waukesha, WI). High-resolution, oblique axial T2-weighted images were obtained (field of view =26 cm2; in-plane resolution = 0.7 x 1.0 mm; slice thickness = 2.4 mm; echo time = 109.8 ms; repetition time = 3 s) during the clinical prostate MR protocol. Three DWI sequences were acquired: 1) ‘standard’ ssEPI; 2) rFOV; 3) msEPI (acquisition parameters are shown in Table 1). Images of msEPI DWI were reconstructed with the phase-corrected multiplexed sensitivity encoding (MUSE) method9. ADC maps of different diffusion series were calculated as follows
$$ADC = \frac{\ln(I_{b100} - I_{b800})}{800 - 100}$$
with Ib800 and Ib100 denoting the signal intensity at b=800 and b=100, respectively.
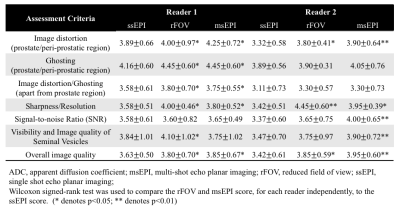
Image quality assessment: Independent, blinded review of the three DWI series was completed by a board certified radiologist and diagnostic radiology trainee (6 and 4 years of clinical prostate MR experience, respectively). Oblique axial T2-weighted images and ADC maps were available to the readers for reference. Image cropping to an identical field of view maintained the blinded nature of image assessment. DWI image quality was evaluated on a 5-point scale (1 lowest, 5 best possible score) for multiple criteria described in Table 2.
Quantitative ADC assessment:
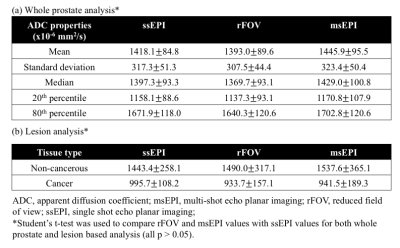
Whole prostate analysis: Whole prostate contouring was completed using the b=100 s/mm2 images. Summary statistics for ADC analysis included mean, standard deviation, median, 20th percentile, and 80th percentile.
MR-fusion biopsy lesion analysis: For a subset of patients who received MR-fusion biopsy, the ADC values of MR target lesions were analyzed using a co-localized and uniformly sized ROI. An identical ‘non-cancerous’ ROI was placed in a region of the contralateral gland that was negative for malignancy on biopsy.
Wilcoxon signed-rank test and Student’s t-test were used as appropriate to test for statistical significance. (p-value ≤ 0.05 = statistically significant).
Results
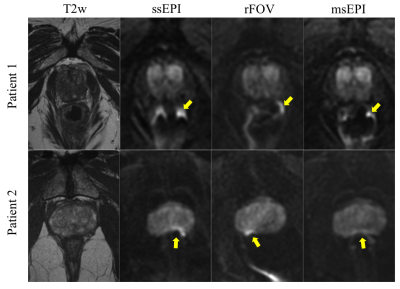
Twenty-five patients were recruited and, after 5 exclusions (incorrect DWI prescription), 20 male patients were included. The average age was 63.9 years (range, 44-76 years) and average PSA was 6.55 ng/mL (range, 1.13-10.84 ng/mL). This included patients with biopsy proven prostate cancer (n= 9), patients currently in active surveillance (n= 4), patients without biopsy proven cancer but elevated PSA (n= 6), and one patient with biochemical recurrence after high intensity focused ultrasound treatment. Seven patients had MR fusion biopsies with all analyzed targets positive for cancer.Image quality results: Both rFOV and msEPI reached higher qualitative scores, compared to ssEPI, in multiple measures. This difference was statistically significant for both readers in prostate/peri-prostatic distortion, sharpness/resolution, and overall image quality (Table 2; Figure 1). At least one of the two readers demonstrated statistically greater image quality, for both rFOV and msEPI, in the remaining criteria (Table 2).
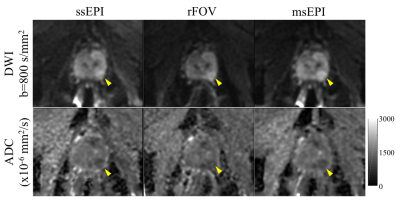
Quantitative ADC results: There was no significant difference across acquisitions in whole prostate ADC values, including mean, median, standard deviation, 20th percentile, or 80th percentile (Table 3a). Importantly, no significant difference in ADC measurements was observed across acquisitions in biopsy-confirmed cancer or comparison non-cancerous tissue (Table 3b; Figure 2).
Discussion
Prospective comparison of rFOV and msEPI to ssEPI, in either biopsy proven or suspected prostate cancer patients, demonstrated that both rFOV and msEPI reduce distortion artifacts, and improve image quality, in comparison to ssEPI. Further, comparable ADC maps and ROI-based measurements are obtained across all three acquisitions.It is known that ssEPI DWI of the prostate can suffer severe image distortion due to susceptibility-related field in-homogeneities, often related to the neighboring air-filled rectum. Distortion reduction techniques, rFOV and msEPI, allow for a shorter readout time or optimized readout direction and thus lead to reduced distortion and ghosting9-10.
In summary, rFOV and msEPI showed improved image quality while maintaining reproducible ADC quantification. While the image quality benefits of rFOV have been suggested previously11-12, resulting in some early adoption into clinical practice, msEPI is a more novel, and less well studied technique. Furthermore, the results of this study demonstrating reproducible ADC quantification may have important implications for research generalizability and comparison of repeat clinical imaging.
Acknowledgements
The authors wish to acknowledge GE Healthcare and Bracco Diagnostics who provide research support to the University of Wisconsin.References
1. Padhani AR, Liu G, Mu-Koh D, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11:102-125.
2. Bammer, R. Basic principles of diffusion-weighted imaging. European journal of radiology 2003;45(3):169-184.
3. Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, Allen C, Emberton M, Moore CM, Kasivisvanathan V. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol 2019; doi: 10.1038/s41585-019-0212-4. [Epub ahead of print]
4. Lawrence EM, Gallagher FA, Barrett T Warren AY, Priest AN, Goldman DA, Sala E, Gnanapragasam VJ. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. Am J Roentgenol 2014;203(3):W280-6.
5. Salami SS, Ben-Levi E, Yaskiv O, Turkbey B, Vilani R, Rastinehad AR. Risk stratification of prostate cancer utilizing apparent diffusion coefficient value and lesion volume on multiparametric MRI. J Magn Reson Imaging 2017;45(2):610-616.
6. Lawrence EM, Tang SY Barrett T, Koo B, Goldman DA, Warren AY, Axell RG, Doble A, Gallagher FA, Gnanapragasam JV, Kastner C, Sala E. Prostate cancer: performance characteristics of combined T2W and DW-MRI scoring in the setting of template transperitoneal re-biopsy using MR-TRUS fusion. Eur Radiol. 2014;24(7):1497-1505.
7. Bjurlin MA, Rosenkrantz AB, Sarkar S, Lepor H, Huang WC, Huang R, Venkataraman R, Taneja SS. Prediction of Prostate Cancer Risk Among Men Undergoing Combined MRI-targeted and Systematic Biopsy Using Novel Pre-biopsy Nomograms tha incorporate MRI findings. Urology 2018;112:112-120.
8. Tamada T, Prabhu V, Li J, Babb JS, Taneja SS, Rosenkrantz AB. Prostate Cancer: Diffusion-weighted MR Imaging for Detection and Assessment of Aggressiveness-Comparison between Conventional and Kurtosis Models. Radiology 2017;284:100-108.
9. Chen NK, Guidon A, Chang HC, & Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41-47.
10. Zhang Y, Holmes J, Rabanillo I, Guidon A, Wells S, Hernando D. Quantitative diffusion MRI using reduced field-of-view and multi-shot acquisition techniques: Validation in phantoms and prostate imaging. Magnetic resonance imaging 2018;51:173-181.
11. Korn N, Kurhanewicz, Banerjee S, Starobinets O, Saritas E, Noworolski. Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection. Magnetic resonance imaging 2015;33(1):56-62.
12. Feng Z, Min X, Sah VK, Li L, Cai J, Deng M, Wang L. Comparison of field-of-view (FOV) optimized and constrained undistorted single shot (FOCUS) with conventional DWI for the evaluation of prostate cancer. Clinical Imaging 2015;39:851-855.
Figures