2421
Multi-site analysis of DWI metrics for differentiating pathologically confirmed prostate cancer: patient and digital reference object results1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 4Radiology, University of Michigan, Ann Arbor, MI, United States, 5Radiation Oncology, University of Michigan, Ann Arbor, MI, United States, 6Radiology, Brigham and Womens Hospital, Boston, MA, United States, 7Neuroimaging Research, Barrow Neurological Institute, Phoenix, AZ, United States, 8Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 9Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 10Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 11Institute for Computer Engineering and Sciences, University of Texas, Austin, TX, United States, 12radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 13Advanced Imaging Research Center, Oregon Health Sciences University, Portland, OR, United States, 14Radiology and Neurology, University of Washington, Seattle, WA, United States, 15Radiology, Johns Hopkins University, Baltimore, MD, United States, 16Urological Surgery, Medical College of Wisconsin, Milwaukee, WI, United States, 17Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 18Pathology, Chiang Mai University, Chiang Mai, Thailand
Synopsis
This study presents a multi-site study measuring the ability of site-specific diffusion weighted imaging fitting algorithms for differentiating prostate cancer. A dataset of DWI collected from 33 patients and a simulated digital reference object was distributed to thirteen sites who fit the multi-b DWI models with onsite-implemented software. Derived parametric maps were then submitted for central analysis. Each map was aligned to the T2-weighted image, and DWI metrics were extracted from aligned pathologist annotations. A statistical analysis was performed to determine the ability of each metric to differentiate PCA and to determine how fits of simulated data differed between sites.
Introduction
One in seven men will be diagnosed with prostate cancer. Not all cases have lethal potential, and ongoing radiological studies are aimed at better differentiating aggressive from indolent disease. Rad-path correlation is useful for validating imaging technology, where ‘gold-standard’ pathologist annotations are compared to imaging biomarkers. Diffusion weighted imaging (DWI) is commonly used for diagnosing prostate cancer and DWI is weighted heavily as a deciding factor in the PIRADS grading scale for radiographic diagnosis1. Quantitative calculation of diffusion values can however vary due to the software implementation of mathematical fits. This study compares the quantitative DWI parameters derived from software developed or implemented at 13 collaborating sites when applied to a common data set of prostate imaging with pathological correlation, and two DROs developed for kurtosis and bi-exponential fitting models. We compare the DWI parameters calculated to determine whether cancer detection is impacted by algorithm implementation and fit parameters.Methods
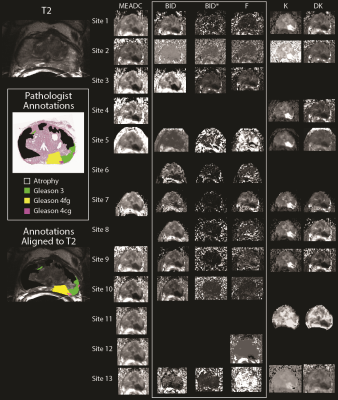
Patient Population and Data Acquisition Thirty-three PCA patients undergoing prostatectomy were recruited for this institutional review board (IRB) approved study. Patients underwent MP-MRI prior to prostatectomy on a 3T MRI scanner (General Electric, Waukesha, WI) using an endorectal coil. MP-MRI included field-of-view (FOV) optimized and constrained undistorted single shot (FOCUS) diffusion weighted imaging (DWI) with ten b-values (b=0, 10, 25, 50, 80, 100, 200, 500, 1000, and 2000) and T2-weighted imaging. Robotic prostatectomy was performed, and prostate samples were sectioned using patient-specific custom 3D printed slicing jigs to match the slice orientation to the T2 weighted image2,3.Ground Truth Cancer Localization Prostate samples were whole-mount hematoxylin and eosin (H&E) stained, digitized, and annotated by a urological fellowship trained pathologist (Figure 1). A total of 169 slides were included in this study. Annotations of different Gleason patterns were brought into MRI space using a non-linear transform, calculated from control points manually placed2,3. Pathologist-annotated regions (PA-ROIs) that consisted of at least 200 contiguous voxels were included for further analysis, which resulted in 231 cancer (CA) regions of interest (ROIs), and 564 ROIs not associated with cancer (NCA).
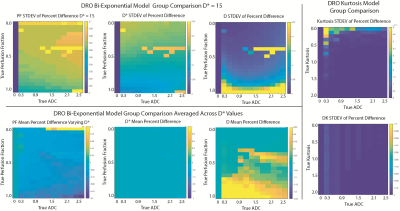
Digital Reference Objects (DRO) Two DROs were created to simulate noiseless magnitude DW images for bi-exponential, and kurtosis (K) diffusion models using a range of tissue-relevant ADC and model-specific values (D*, PF, K). Sites were instructed to apply their fitting and submit results for group comparison.
Diffusion Signal Fitting FOCUS DWI datasets from the PCA patients were de-identified and distributed to collaborating sites. Each site was asked to calculate diffusion parameters using locally developed or implemented software, to fit the b-values with common models. This included a mono-exponential (ME) fit (parameter: MEADC), diffusion kurtosis (parameters: kurtosis (K), and diffusion (DK))4, and a bi-exponential fit (parameters: diffusion (BID), pseudo-diffusion (BID*) and perfusion fraction (F))5. Each site submitted the calculated maps back to the primary institution for comparative analysis. Once submitted, site results were pre-processed by the coordinating site to ensure each map was in a common space and scaled properly to the same units. Each map was then re-sliced and resampled into the T2 space for comparison to the pathologist annotated ROIs.
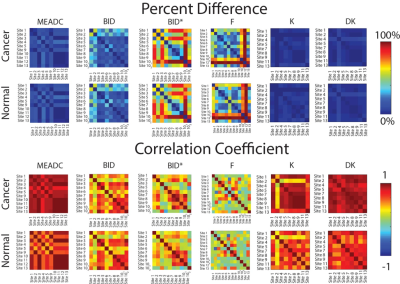
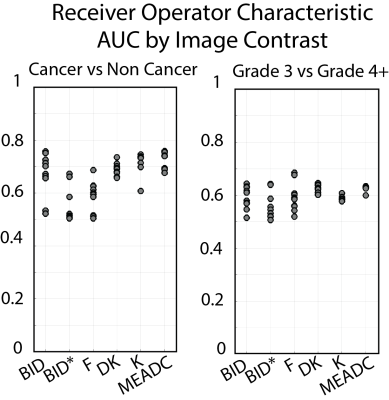
Statistical Analysis: Patient Data Median diffusion values were calculated within each ROI and concatenated into a matrix for further statistical analysis. Each parameter was compared across sites within non-cancer (NCA) and PCA ROIs to determine inter-site correlation and percent difference. A receiver operator characteristic (ROC) analysis was performed to determine the ability of each metric (and each site) to differentiate regions of cancer from normal tissue, as well as low-grade from high-grade cancer.
Statistical Analysis: DRO The percent difference was calculated voxel-wise between sites for each parameter. To visualize the results, the standard deviation of the percent difference was mapped.
Results
A representative image from one patient is shown in Figure 1 with site specific submissions of DWI parametric maps. Comparison between sites indicated that K, DK, and MEADC were most stable (Figure 2). Bi-exponential parameters varied more substantially between sites. Correlation between sites followed the same trend (Figure 2 Bottom). When assessing parameters for cancer differentiation, the AUCs associated with MEADC, K, and DK varied the least between sites (Figure 3). The DRO results indicated minimal discordance between sites (Figure 4).Discussion
We present a multi-site study quantifying the differences in diffusion fitting algorithms for differentiating prostate cancer. We find that K, DK and MEADC are the most reliably calculated parameters across sites, and most reliably differentiate prostate cancer. In simulated, noiseless data, minimal discordance was measured, indicating cross site variability is heavily influenced by real world factors. This study demonstrates that contrast between PCA and NCA is maintained independent of the site-specific implementation K, DK, and MEADC fitting.Acknowledgements
Advancing a Healthier Wisconsin, the State of Wisconsin Tax Check off Program for Prostate Cancer Research, National Center for Advancing Translational Sciences, NIH UL1TR001436, TL1TR001437, R01CA218144, U01CA176110, R21CA231892, U01CA166104, R01CA190299, P01CA085878, U24CA180918, R01CA160902, U01CA151261, R01CA158079, U01CA172320, U01CA211205, P30CA008748, U01CA142565, U01CA207091, U01CA154602, R50CA211270, 5P30CA006973 (Imaging Response Assessment Team-IRAT), U01CA140204, U01CA183848, and R01CA221938.References
1. Vargas HA, Hotker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. European radiology. 2016;26(6):1606-1612.
2. Hurrell SL, McGarry SD, Kaczmarowski A, et al. Optimized b-value selection for the discrimination of prostate cancer grades, including the cribriform pattern, using diffusion weighted imaging. J Med Imaging (Bellingham). 2018;5(1):011004.
3. McGarry SD, Hurrell SL, Iczkowski KA, et al. Radio-pathomic Maps of Epithelium and Lumen Density Predict the Location of High-Grade Prostate Cancer. Int J Radiat Oncol Biol Phys. 2018;101(5):1179-1187.
4. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-1440.
5. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497-505.
Figures