2418
The Value of Obtaining Incremental Cores in MRI Guided Prostate Biopsy1School of Medicine, Case Western Reserve University, Cleveland, OH, United States, 2Urology Institute, University Hospitals, Cleveland, OH, United States, 3Radiology, Mayo Clinic, Rochester, MN, United States, 4Department of Diagnostic, Interventional and Pediatric Radiology, Bern University Hospital, Bern, Switzerland, 5Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 6Radiology, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, United States, 7Radiology, Mayo Clinic, Scottsdale, AZ, United States
Synopsis
The value of incremental biopsy cores during in-gantry MRI-guided targeted prostate biopsy for prostate cancer diagnosis was examined. 135 patients with 175 lesions underwent in-gantry targeted prostate biopsy and the rates of pathology upgrading for sequential cores were determined. Significant upgrading was observed when increasing from 1 to 2 cores and from 2 to 3 cores, but not from core 3 to 4. Higher Prostate Imaging Reporting and Data System (PI-RADS) Version 2.0 score and anteriorly located lesions were more likely to result in an upgrade.
Introduction
Prostate cancer is the leading cancer diagnosis and the second leading cause of cancer death in men in the United States.1, 2 Traditionally, increased PSA values or suspicious digital rectal exam are indications for prostate biopsy, which is required for diagnosis of prostate cancer.3, 4 Systematic biopsies, which have been shown to both under-detect clinically significant prostate cancer and over-detect clinically insignificant cancer, have been performed as the mainstay.5 During systematic biopsies, typically 12 cores are obtained, spaced throughout the prostatic parenchyma using ultrasound guidance.1, 3 The use of multiparametric MRI (mpMRI) has allowed for the localization of suspicious lesions, and MRI-targeted biopsies have emerged as a technique that both improves prostate cancer detection and minimizes the number of samples required.1, 5, 6, 7 In-gantry MRI-guided targeted prostate biopsy (MRGB) has advantages over other targeted biopsy methods, including improved visualization of the needle trajectory in relation to the lesion and accurate documentation that the lesion was indeed sampled.1 While MRGBs have reduced the number of cores required during prostate biopsies, an ideal acceptable number of cores has yet to be determined. A 2016 consensus statement by the American Urological Association and the Society of Abdominal Radiology recommends that at least two cores be obtained per lesion during MRGB in men with prior negative biopsies and more cores may be obtained at the discretion of the physician.8 The ideal number of cores to obtain is essential knowledge in order to optimize the diagnosis of clinically significant prostate cancer while minimizing complications.9Methods
In this Institutional Review Board approved retrospective study, 136 consecutive patients with 175 lesions undergoing in-gantry MRGB between October 2016 and May 2019 were included for analysis. Patients were stratified into subgroups with at least 2, at least 3 and at least 4 biopsy cores per lesion. The 2014 International Society of Urological Pathology Grading (ISUPG) system was used to describe the biopsy results. Clinically significant cancer was defined as an International Society of Urological Pathology Grade (ISUPG) of 2 or greater. Upgrading between sequential cores was defined as a greater ISUPG for the second core compared to the first core when comparing cores 1 and 2, a greater ISUPG for the third core compared to the first and second cores when comparing cores 2 and 3, and a greater ISUPG for the fourth core compared to the first, second and third cores when comparing cores 3 and 4. The Chi-square test was used for categorical data.Results
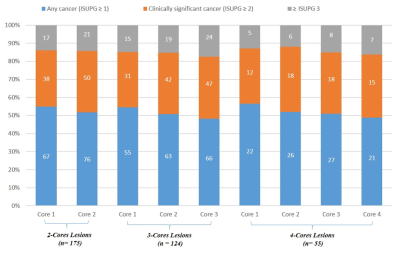
Our results demonstrated a rate of upgrades of ISUPG of 12.6% (22 of 175), 11.3% (14 of 124) and 1.9% (1 of 52) when the number of cores increased from 1 to 2, 2 to 3, and 3 to 4 respectively. Of these upgrades, an increase from clinically insignificant (ISUPG ≤ 1) to clinically significant prostate cancer (ISUPG ≥ 2) was found in 6.9% (12 of 175), 4% (5 of 124), and 0% (0 of 52) when the number of cores increased from 1 to 2, 2 to 3, and 3 to 4 respectively. An increase in upgrade rate was observed with increasing Prostate Imaging Reporting and Data System (PI-RADS) Version 2.0 score and with anterior location, but not with lesion size. Upgrade rates when increasing from 1 to 2 cores were 6.5% (4 of 62), 10.8% (7 of 65) and 22.9% (11 of 48) for PI-RADS 3, 4 and 5 respectively (p = 0.01). When increasing from 2 to 3 cores, upgrade rates were 2.7% (1 of 37), 10.9% (5 of 46) and 19.5% (8 of 41) for PI-RADS 3, 4 and 5 respectively (p = 0.01).Discussion
Our results show that increasing the number of cores obtained per lesion from 1 to 2 and from 2 to 3 results in an increase in the clinical significance of prostate cancer diagnosis. The results did not show an increase in clinically significant cancer diagnosis when increasing from 3 to 4 cores. These results, along with low complication rates, indicate that obtaining 3 cores per lesion is sufficient to make an accurate diagnosis while a 4th core provides little utility. According to National Comprehensive Cancer Network (NCCN) there are significant differences in the recommended management of prostate cancer that is ISUPG 1 (very low or low risk) and prostate cancer that is ISUPG 2 or 3 (favorable or unfavorable intermediate risk).10 Our results show a 4% upgrade rate when increasing from 2 to 3 cores from ISUPG 1 to 2 and from ISUPG 2 to 3. These upgrades in ISUPG mean a patient is more likely to be recommended to receive invasive treatment such as radical prostatectomy or external beam radiation therapy rather than to be placed on active surveillance.10 The small but significant increase in prostate cancer diagnoses when increasing the number of cores from 1 to 2 and from 2 to 3 warrants that 3 cores be obtained per index lesion in order to avoid missing a clinically significant cancer diagnosis and the under-treatment of prostate cancer.Conclusion
Increasing the number of cores from 1 to 2 and from 2 to 3 results in a higher detection rate of clinically significant cancers.Acknowledgements
No acknowledgement found.References
1. Verma S, Choyke PL, Eberhardt SC, Oto A, Tempany CM, Turkbey B, Rosenkrantz AB. The Current State of MR Imaging-targeted Biopsy Techniques for Detection of Prostate Cancer. Radiology. 2017 Nov;285(2):343-356. doi: 10.1148/radiol.2017161684. Review. PubMed PMID: 29045233; PubMed Central PMCID: PMC5673043.
2. Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012 Apr;30(2):195-200. doi: 10.1007/s00345-012-0824-2. Epub 2012 Apr 5. Review. PubMed PMID: 22476558.
3. Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA.2017 Jun 27;317(24):2532-2542. doi: 10.1001/jama.2017.7248. Review. PubMed PMID: 28655021.
4. Uriburu-Pizarro F, Kasivisvanathan V, Puech P, Villers A. Pre-biopsy MRI as an adjunct for cancer detection in men with elevated PSA and no previous biopsy. Transl Androl Urol. 2017 Jun;6(3):387-394. doi: 10.21037/tau.2017.01.19. Review. PubMed PMID: 28725580; PubMed Central PMCID: PMC5503968.
5. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018 May 10;378(19):1767-1777. doi: 10.1056/NEJMoa1801993. Epub 2018 Mar 18. PubMed PMID: 29552975.
6. Porpiglia F, De Luca S, Passera R, De Pascale A, Amparore D, Cattaneo G, Checcucci E, De Cillis S, Garrou D, Manfredi M, Mele F, Bollito E, Fiori C. Multiparametric Magnetic Resonance/Ultrasound Fusion Prostate Biopsy: Number and Spatial Distribution of Cores for Better Index Tumor Detection and Characterization. J Urol. 2017 Jul;198(1):58-64. doi: 10.1016/j.juro.2017.01.036. Epub 2017 Jan 16. PubMed PM
7. Elkhoury FF, Simopoulos DN, Marks LS. Targeted Prostate Biopsy in the Era of Active Surveillance. Urology. 2018 Feb;112:12-19. doi: 10.1016/j.urology.2017.09.007. Epub 2017 Sep 27. Review. PubMed PMID: 28962878; PubMed Central PMCID: PMC5856576.
8. Rosenkrantz AB, Verma S, Choyke P et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 2016;196(6):1613–1618
9. Zhang M, Milot L, Khalvati F, Sugar L, Downes M, Baig SM, Klotz L, Haider MA. Value of Increasing Biopsy Cores per Target with Cognitive MRI-targeted Transrectal US Prostate Biopsy. Radiology. 2019 Apr;291(1):83-89. doi: 10.1148/radiol.2019180712. Epub 2019 Jan 29. PubMed PMID: 30694165
10. National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (Accessed on July 21, 2019)
Figures