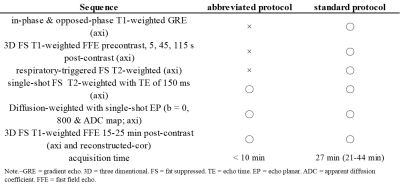
2412
Optimized Colorectal Liver Metastasis Screening: Diagnostic Performance of an Abbreviated Gadoxetic Acid-enhanced MR Imaging Protocol.1Radiology, Gifu University, Gifu, Japan, 2Diagnostic Radiology and Nuclear Medicine, Hamamatsu University School of Medicine, Hamamatsu, Japan, 3Radiology Services, Gifu University Hospital, Gifu, Japan
Synopsis
Gadoxetic acid-enhanced MR imaging is an essential modality for the screening and assessment of hepatic diseases, which has an incremental value when combined with contrast-enhanced computed tomography for detecting colorectal liver metastasis (CLM). We assessed the diagnostic performance of an abbreviated protocol (axial heavily T2-weighted + axial and reconstructed-coronal hepatobiliary phase + axial diffusion-weighted imaging) compared with the standard routine clinical one for CLM screening. Our results demonstrated excellent performance for the detection of CLM comparable to standard one less than one third of acquisition time.
Introduction
Contrast-enhanced computed tomography (CECT), in general, is first-choice imaging modality for the assessment of metastatic lesions. However, this modality has a low sensitivity and specificity for the detection and particularly characterization of liver lesions1. Over the 15 years since gadoxetic acid-enhanced magnetic resonance (MR) imaging was first introduced in daily clinical practice, it has become an essential modality for the screening and assessment of hepatic diseases and is recommended in several clinical guidelines2,3. The hepatobiliary phase imaging is especially excellent for the differentiation between lesions with and without functional hepatocytes4, which has an incremental value when combined with CECT for detecting additional liver metastases, especially in colorectal cancer5-7. Nevertheless, MR imaging is still associated with long examination and interpretation times, which increases the costs and limits its routine use as a surveillance-imaging tool. In order to make MR imaging more accessible, there has been an increasing interest in developing abbreviated MR imaging protocols, which have been applied in hepatocellular carcinoma (HCC) and colorectal liver metastasis (CLM) surveillance8-11. The purpose of this study was to evaluate the diagnostic performance of an abbreviated gadoxetic acid-enhanced MR imaging protocol compared with the standard one for CLM screening.Materials and Methods
This retrospective study was approved by our institutional review board and written informed consent was waived. Between May 2017 and July 2019, a total of 68 consecutive patients (40 men, 28 women; mean age, 63.9 years, range, 22-84 years) with colorectal cancer underwent gadoxetic acid-enhanced MR imaging for CLM surveillance using a 3-Tesla (T) clinical scanner with a 32-channel torso coil (n = 42; Ingenia CX; Philips Healthcare, Best, The Netherlands), a 3-T with a 16-channel (n = 22; Intera Achieva Quasar Dual), or a 1.5-T with a 20-channel (n = 4; Ingenia Prodiva CX). The standard MR imaging protocol consisted of the following sequences: axial in-phase and opposed-phase T1-weighted gradient-echo imaging; axial breath-hold three-dimensional fat-suppressed T1-weighted fast field-echo (3D-FS-T1W) imaging; axial respiratory-triggered two-dimensional fat-suppressed T2-weighted turbo spin-echo imaging (FS-T2WI); axial breath-hold single-shot FS-T2WI with TE of 150 msec (heavily T2WI); and axial respiratory-triggered two-dimensional diffusion-weighted imaging (DWI) with a single-shot echo-planar sequence. Contrast-enhanced dynamic images were obtained after 0.025 mmol/kg body weight (0.1 mL/kg) of gadoxetic acid were administered with 30 mL saline flush at a rate of 1 mL/s. The hepatobiliary phase (HBP) images were obtained 16 min (range, 15-25 min) after an intravenous injection of gadoxetic acid. Image set 1 as an abbreviated protocol included axial heavily T2WI, axial and reconstructed-coronal 3D-FS-T1W HBP sequences, and axial DWI (Table 1). Image set 2 consisted of the standard protocol as above. Two radiologists independently evaluated two image sets with a week time interval and recorded on the number, location, and size of focal liver lesions (FLLs). Additionally, qualitative ratings in each FLLs were recorded on a 5-point scale (1 = definitely benign, 2 = probably benign, 3 = indeterminate, 4 = probably malignant, and 5 = definitely malignant). A FLL with score 4 or 5 was defined as malignant (CLM). A maximum of 5 FLLs per patient were recorded. A total of 166 FLLs (mean maximum diameter, 16.0 mm; range, 3-64 mm) without simple cyst were detected. One hundred three (19.9 mm; 3-64 mm) of 166 FLLs were malignant, 47 of which were a pathologically proven by means of liver resection. Remaining 63 FLLs (9.7 mm; 4-27 mm) were benign (hemangioma, n = 40; complicated cyst and hyperplastic nodule, n = 8, respectively; focal nodular hyperplasia, hepatic adenoma, and focal hepatopathy, n = 2, respectively; hepatic abscess, n = 1). All available follow-up studies were used to provide collect diagnosis of all FLLs without histopathological examination. Sensitivity, positive predictive value (PPV), and areas under the curve (AUCs) were compared to assess the diagnostic values in differentiating malignant and benign FLLs. Weighted kappa analysis was conducted for intra- and inter-reader variability.Results
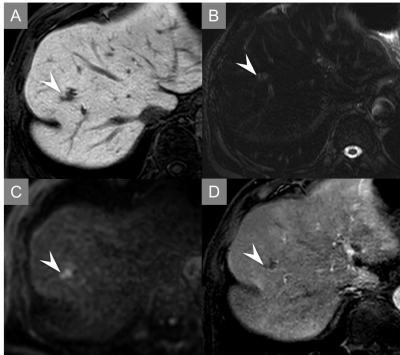
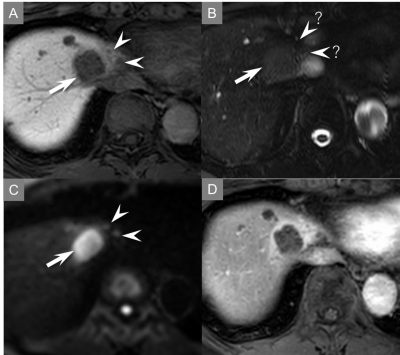
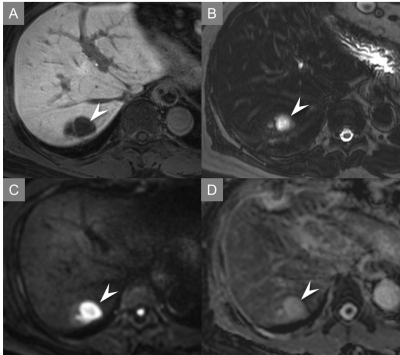
Quantitative results were demonstrated in Table 2. The sensitivity and PPV for the differentiating malignant and benign FLLs in abbreviated protocol were very high (almost 90%) for both readers (Fig. 1-3). No statistically significant differences in sensitivity, PPV, and AUCs were found between both protocols. The intra-reader agreement between the abbreviated protocol and standard one was 0.835 in reader 1 and 0.901 in reader 2, indicating perfect agreement. The inter-reader agreement for the abbreviated protocol was 0.693, indicating substantial agreement.Discussion
Our proposed abbreviated protocol demonstrated excellent performance for the differentiating malignant and benign FLLs comparable to standard one less than one third of acquisition time. Canellas et al.10 reported an abbreviated MR imaging protocol for CLM surveillance, however their protocol, which included a breath-hold coronal ultrafast spin-echo sequence, was different from ours. Our protocol, which included a heavily T2WI sequence, was consisted of routine clinical practice sequence for FLLs. Sensitivity for the differentiating malignant and benign FLLs in both readers was adequately high but slightly inferior to the previous study12. This was because CLMs missed to detect were relatively small (4.5 mm; 3-5 mm) and in sight of blood vessels or liver margin.Conclusion
Our proposed abbreviated protocol is fast alternative to the standard one and has comparable performance for the differentiating malignant and benign FLLs.Acknowledgements
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.References
1. Tirumani SH, Kim KW, Nishino M, et al. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics : a review publication of the Radiological Society of North America, Inc 2014;34:1908-28.
2. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology 2014;87 Suppl 1:7-21.
3. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatology international 2017;11:317-70.
4. Sano K, Ichikawa T, Motosugi U, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 2011;261:834-44.
5. Kim HJ, Lee SS, Byun JH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radiology 2015;274:712-22.
6. Scharitzer M, Ba-Ssalamah A, Ringl H, et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. European radiology 2013;23:2187-96.
7. Sofue K, Tsurusaki M, Murakami T, et al. Does Gadoxetic acid-enhanced 3.0T MRI in addition to 64-detector-row contrast-enhanced CT provide better diagnostic performance and change the therapeutic strategy for the preoperative evaluation of colorectal liver metastases? European radiology 2014;24:2532-9.
8. Lima PH, Fan B, Berube J, et al. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR American journal of roentgenology 2019:1-9.
9. Khatri G, Pedrosa I, Ananthakrishnan L, et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. Journal of magnetic resonance imaging : JMRI 2019.
10. Canellas R, Patel MJ, Agarwal S, Sahani DV. Lesion detection performance of an abbreviated gadoxetic acid-enhanced MRI protocol for colorectal liver metastasis surveillance. European radiology 2019;29:5852-60.
11. Canellas R, Rosenkrantz AB, Taouli B, et al. Abbreviated MRI Protocols for the Abdomen. Radiographics : a review publication of the Radiological Society of North America, Inc 2019;39:744-58.
12. Muhi A, Ichikawa T, Motosugi U, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. Journal of magnetic resonance imaging : JMRI 2011;34:326-35.
Figures