2409
Can IVIM Be used for Preoperative Assessment of Microvascular Invasion in Hepatocellular Carcinoma ?1West China Hospital, Sichuan University, Chengdu, China, 2GE Healthcare, MR Research, Beijing, China
Synopsis
Microvascular invasion (MVI) is one of the most important factors for the recurrence of hepatocellular carcinoma (HCC), however, accurate preoperative evaluation of MVI is quietly difficult because of the controversy results caused by the conventional imaging features. Compared with diffusion-weighted imaging (DWI), Intravoxel incoherent motion (IVIM) diffusion-weighted MR imaging could better characterize heterogeneity and irregularity of tissue components, and thus may have the potential to better evaluate MVI. In this study, we prospectively determine the usefulness of IVIM parameters and conventional radiologic features for preoperative prediction of MVI in patients with HCC.
Purpose
To prospectively evaluate the potential role of IVIM and conventional radiologic features for preoperative prediction of MVI in patients with HCC.Materials and Methods
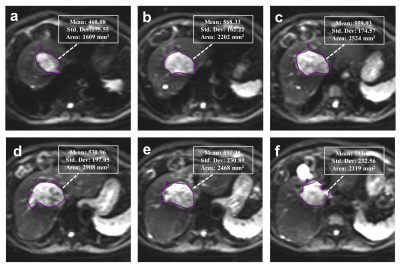
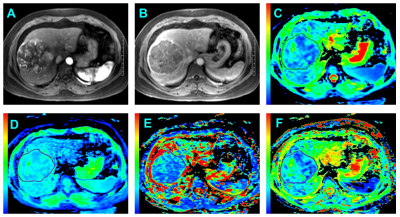
Institutional review board approval and written informed consent were obtained for this study. A cohort comprising 115 patients with 135 newly diagnosed HCCs between January 2016 and April 2017 were evaluated. For all examinations, studies were carried out by using a 3.0 T MR system (Discovery MR 750, GE Healthcare, Milwaukee, USA). A sixteen-channel phased-array torsor coil (GE Medical System) was used for all measurements. IVIM was performed by using an echo-planner imaging sequence with respiratory gating, the parallel imaging was used to short the scanning time and reduce image distortion. The IVIM-DW MR imaging was performed before the injection of contrast agents. Twelve b values from 0 to 1000 sec/mm2 (0, 10, 20, 40, 80, 100, 150, 200, 400, 600, 800 and 1000 sec/mm2) were obtained, and the number of excitations (NEX) for each b value was 1, 6, 4, 2, 2, 2, 1, 1, 2, 4, 6 and 6, respectively. All the IVIM-DW images were analyzed by two independent radiologists blindly, the whole tumor volume was selected for the region of interest (ROI) measurement (Figure 1). Each radiologist drew freehand ROI to outline the tumor on the original DW images (b=400) on each tumor slice, and try to avoid the hemorrhage, calcified and necrotic areas. The ADC, ADCslow, ADCfast and f value were automatically calculated by the workstation, and the averaged value of all tumor slices of each parameter was used for further statistical analyze. Interobserver agreement were checked, univariate and multivariate logistic regression were used for screening the risk factors. Receiver operating characteristics (ROC) curves analyses were performed to evaluate the diagnostic performance.Results
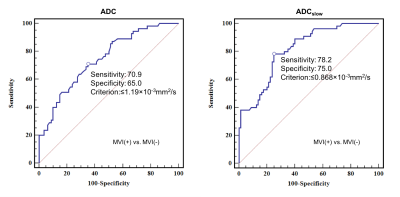
Good to perfect interobserver agreement was found between two observers regarding the imaging features (all Kappa >0.7). The ADC value measured by both two radiologists were significant higher in the MVI-Negative group than the MVI-Positive group (All p <0.001). In addition, the ADCslow value were also significant higher in the MVI-Negative group compared with the MVI-Positive. No statistical significance was obtained from the ADCfast (R1: p=0.103; R2: p=0.093) and f (R1: p=0.745; R2: p=0.724) in those patients with MVI-Positive (Figure 2) compared with MVI-Negative. Features significantly related to MVI of HCC at univariate analysis were reduced ADC (odds ratio, 0.341; p<0.001), ADCslow (odds ratio, 0.141; p<0.001) and irregular circumferential enhancement (odds ratio, 9.908; p<0.001). At multivariate analysis, only ADCslow (odds ratio, 0.096; p<0.001) was the independent risk factor for MVI of HCC. The mean ADCslow value for MVI of HCC showed an area under ROC curves of 0.815 (95% CI: 0.740-0.877) (Figure 3).Conclusion and Discussion
In the present study, the difference of diffusion parameters and the radiologic features were evaluated between the MVI-Positive and MVI-Negative groups and all the risk factors were further screened by using univariate and multivariate logistic regression analysis. The results demonstrated that the decreased ADC and ADCslow value were significantly with the presence of MVI in HCC at univariate analysis. Furthermore, the multivariate analysis suggested that only the ADCslow based on the IVIM model was the risk factor for MVI and which yield better diagnostic performance in comparison with ADC derived from the mono-exponential model. Thus, the results of the preliminary study have demonstrated that the decreased ADCslow value was independent risk factor for predicting MVI of HCC.Acknowledgements
None.References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87-108.
2. Kudo M, Trevisani F, Abou-Alfa GK, Rimassa L (2016) Hepatocellular Carcinoma: Therapeutic Guidelines and Medical Treatment. Liver Cancer 6:16-26.
3. Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379:1245-1255.
4. Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K (2016) Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 25:24-29.
5. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK (2013) A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 20:325-339.
6. Witjes CD, Willemssen FE, Verheij J et al (2012) Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging 36:641-647.
7. Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA (2007) Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg 245:435-442.
8. Ramos Rubio E, Llado Garriga L (2010) [Usefulness of pre-surgical biopsy in selecting patients with hepatocellular carcinoma for liver transplant]. Cir Esp 87:133-138.
9. Hirokawa F, Hayashi M, Miyamoto Y et al (2014) Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res 44:846-853.
10. Renzulli M, Brocchi S, Cucchetti A et al (2016) Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology 279:432-442.
11. Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B (2018) Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol 24:2348-2362.
12. Wei Y, Gao F, Wang M et al (2018) Intravoxel incoherent motion diffusion-weighted imaging for assessment of histologic grade of hepatocellular carcinoma: comparison of three methods for positioning region of interest. 10.1007/s00330-018-5638-1.
13. Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J (2014) Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 29:330-336.
14. Valerio M, Zini C, Fierro D et al (2016) 3T multiparametric MRI of the prostate: Does intravoxel incoherent motion diffusion imaging have a role in the detection and stratification of prostate cancer in the peripheral zone? Eur J Radiol 85:790-794.
15. Marzi S, Piludu F, Sanguineti G et al (2017) The prediction of the treatment response of cervical nodes using intravoxel incoherent motion diffusion-weighted imaging. Eur J Radiol 92:93-102.
16. Iima M, Le Bihan D (2016) Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 278(1):13-32.
17. Meeus EM, Zarinabad N, Manias KA et al (2018) Diffusion-weighted MRI and intravoxel incoherent motion model for diagnosis of pediatric solid abdominal tumors. J Magn Reson Imaging 47:1475-1486.
18. Nougaret S, Vargas HA, Lakhman Y et al (2016) Intravoxel Incoherent Motion-derived Histogram Metrics for Assessment of Response after Combined Chemotherapy and Radiation Therapy in Rectal Cancer: Initial Experience and Comparison between Single-Section and Volumetric Analyses. Radiology 280:446-454.
19. Suo S, Lin N, Wang H et al (2015) Intravoxel incoherent motion diffusion-weighted MR imaging of breast cancer at 3.0 tesla: Comparison of different curve-fitting methods. J Magn Reson Imaging 42:362-370.
20. Shen N, Zhao L, Jiang J et al (2016) Intravoxel incoherent motion diffusion-weighted imaging analysis of diffusion and microperfusion in grading gliomas and comparison with arterial spin labeling for evaluation of tumor perfusion. J Magn Reson Imaging 44:620-632.
21. Song XL, Kang HK, Jeong GW et al (2016) Intravoxel incoherent motion diffusion-weighted imaging for monitoring chemotherapeutic efficacy in gastric cancer. World J Gastroenterol 22:5520-5531.
22. Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI (2014) Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology 270:758-767.
23. Joo I, Lee JM, Han JK, Choi BI (2014) Intravoxel incoherent motion diffusion-weighted MR imaging for monitoring the therapeutic efficacy of the vascular disrupting agent CKD-516 in rabbit VX2 liver tumors. Radiology 272:417-426.
24. Lee Y, Lee SS, Kim N et al (2015) Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology 274:405-415.
25. Amin MB, Edge SB, Greene FL, et al. AJCC cancer Staging Manual. 8th ed. New York: Springer;2017.
26. Jerjir N, Bruyneel L, Haspeslagh M, Quenet S, Coenegrachts K (2017) Intravoxel incoherent motion and dynamic contrast-enhanced MRI for differentiation between hepatocellular adenoma and focal nodular hyperplasia. Br J Radiol 90:20170007.
27. Choi IY, Lee SS, Sung YS et al (2017) Intravoxel incoherent motion diffusion-weighted imaging for characterizing focal hepatic lesions: Correlation with lesion enhancement. J Magn Reson Imaging 45:1589-1598.
28. Klauss M, Mayer P, Maier-Hein K et al (2016) IVIM-diffusion-MRI for the differentiation of solid benign and malign hypervascular liver lesions-Evaluation with two different MR scanners. Eur J Radiol 85:1289-1294.
29. Shirota N, Saito K, Sugimoto K, Takara K, Moriyasu F, Tokuuye K (2016) Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imaging 16:1.
30. Wang WT, Yang L, Yang ZX et al (2018) Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. 286:571-580.
31. Matsui O, Kobayashi S, Sanada J et al (2011) Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 36:264-272.
32. Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL (2014) Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 203:W253-259.
Figures