2406
Analysis of liver function after molecular targeting therapy of HCC with hepatocyte fraction index obtained from gadoxetic acid-enhanced MRI.1Quantum Medical Technology, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan, 2Philips.Japan, Tokyo, Japan, 3Radiology, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan, 4Gastroenterology, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan
Synopsis
Hepatocyte fraction index (HeFra index) obtained from gadoxetic acid-enhanced MR imaging might be possible surrogate marker of liver function after molecular targeting therapy to advanced HCC, and the molecular targeting therapy to advanced HCC do not affect the function of background liver parenchyma.
Purpose
Molecular targeting therapy is widely used for treatment of advanced hepatocellular carcinoma (HCC)1. This novel therapeutic option is expected to block the angiogenesis pathway and restrict the tumor growth. Although this therapy is widely used to the treatment of HCC, the effect to the background liver is not fully understood. The purpose of this prospective study was to elucidate whether the molecular targeting therapy affect the function of background liver parenchyma of advanced HCC with the hepatocyte fraction index (HeFra index)2-4, which is an index for the amount of hepatocyte uptake of gadoxetic acid referenced by splenic enhancement for the estimation of the extracellular space, obtained from gadoxetic acid-enhanced MR imaging.Materials and methods
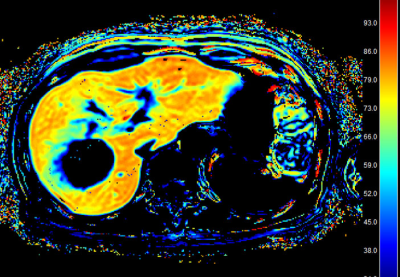
This prospective study was approved by our Institutional Review Board, and written informed consent was obtained from the patients. Ten advanced HCC cases (one female and nine male, average age 73.2±6.2 y.o.) who are planned to perform molecular targeting therapy were included in this study (Lenbatinib N=4, Lenbatinib with intra-arterial administration of anticancer agents N=3, Regrafenib N=2, Sorafenib N=1). Gadoxetic acid-enhanced MR imaging were performed before start of administration of molecular targeting agents (pre-Tx), two weeks and six weeks after started administration of molecular targeting agents (2W-Tx and 6W-Tx) respectively. Therapeutic effect of the agents was assessed with pre-TX and 6W-TX dynamic contrast enhanced MR images of gadoxetic acid-enhanced MRI and the patients were divided into the following two groups, effective group and ineffective group. HeFra index was calculated from ΔR1 values of the liver and spleen with the following formula. ΔR1liver = (1 − φliver) x ΔR1Hepatobiliary + φliver x ΔR1IV+ECS ΔR1spleen = φspleen x ΔR1IV+ECS HeFra index (%) = (ΔR1Hepatobiliary/(ΔR1Hepatobiliary+ΔR1IV+ECS)) x 100 (IV; intravascular, ECS; extracellular space.) Approximately 200mm2 ROIs were set on right anterior, right posterior, left lateral lobe, and spleen, on color map image of HeFra index (Figure 1), carefully devoid of large vessels or bile ducts, focal lesions, and artifacts. Average value of the HeFra index obtained from the three hepatic lobe ROIs were used for this analysis. ALBI score5), which is objective serum liver function index, was also calculated from the serum albumin and bilirubin value on each time points respectively and was compared with HeFra index. Statistical analysis was performed with paired t-test and linear regression analysis, and a P value of < 0.05 was considered statistically significant.Results
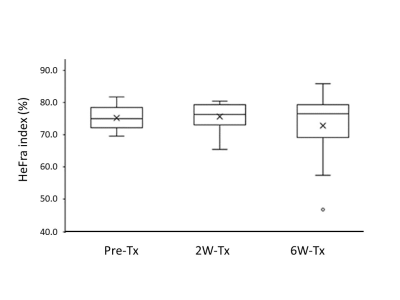
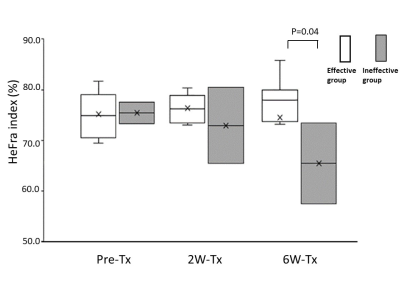
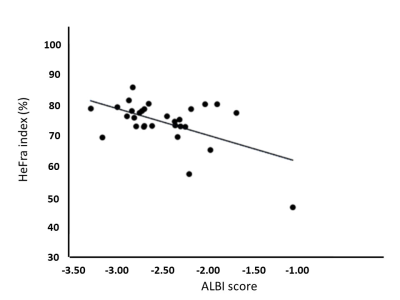
HeFra index and ALBI score of whole patients on pre-Tx, 2W-Tx, 6W-Tx were as follows, HeFra index: 75.2±4.1, 75.7±4.5, 72.7±11.7 (%); ALBI score: -2.58±0.42, -2.40±0.37, -2.33±0.60, respectively (Figure 2). HeFra index (Figure 3) and ALBI score of effective group (N=8) on pre-Tx, 2W-Tx, 6W-Tx were as follows, HeFra index: 75.2±4.5, 76.4±2.8, 74.5±11.8 (%); ALBI score: -2.70±0.28, -2.43±0.36, -2.36±0.68, respectively. HeFra index (Figure 3) and ALBI score of ineffective group (N=2) on pre-Tx, 2W-Tx, 6W-Tx were as follows, HeFra index: 75.4±3.1, 73.0±10.6, 65.5±11.3 (%); ALBI score: -2.09±0.66, -2.26±0.48, -2.23±0.11, respectively. There were no significant differences except ALBI score of Pre-TX and 2W-Tx in effective group, and HeFra index of 2W-Tx and 6W-Tx in ineffective group (p=0.02 and P=0.04 respectively). R value of HeFra index and ALBI score (Figure 4) in whole cases were -0.54 (P=0.002).Discussion
On whole patients’ analysis, there were no significant differences between any combinations of time points both in HeFra index and ALBI score. This results suggests that after molecular targeting therapy of advanced HCC, the background liver function shows no alteration both on imaging biomarker (HeFra index) and serum liver functional marker. Since the HeFra index and ALBI score shows significant negative correlation on linear regression analysis, we supposed that HeFra index might be possible surrogate marker of liver function after molecular targeting therapy to advanced HCC. In detailed analysis, in terms of therapeutic effect of the therapy, ALBI score of Pre-TX (-2.70±0.28) and 2W-Tx (-2.43±0.36) in effective group showed significant difference. Since the increase of ALBI score means worsening of liver function, taking consideration of the cut-off value of the ALBI grade 1 and 2 is -2.60 and ALBI grade 2 and 3 is -1.39, the score increase on Pre-TX and 2W-Tx might be considered as subtle decrease of liver function occured after molecular targeting therapy in effective patients. HeFra index of 2W-Tx (73.0±10.6) and 6W-Tx (65.5±11.3) in ineffective group also showed significant decrease, since the number of the cases in this group is just two, more investigation should be needed to interpret the meaning of this result.Conclusion
In conclusion, with the analysis of HeFra index obtained from gadoxetic acid-enhanced MR imaging, the molecular targeting therapy to advanced HCC do not affect the function of background liver parenchyma. And we suppose that HeFra index might be possible surrogate marker of liver function after molecular targeting therapy to advanced HCC.Summary of main findings
There was no significant difference between pre- and post-treatment hepatocyte fraction. This results suggests that after molecular targeting therapy of advanced HCC, the background liver function shows no alteration on imaging biomarker of liver function.Acknowledgements
No acknowledgement found.References
1) Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018; 15(10): 599-616.
2) Dahlqvist LO, Dahlström N, Kihlberg J, et al. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012; 22(3): 642-653.
3) Noda Y, Goshima S, Okuaki T, et al. Hepatocyte fraction: correlation with noninvasive liver functional biomarkers. Abdom Radiol. 2019 Sep 24. doi: 10.1007/s00261-019-02238-2. [Epub ahead of print] PMID:31552466
4) Ueda Y, Onoda M, Ohno N, et al. 3D hepatocyte fraction index using 3D look locker. Proceedings of the 27th Annual Meeting of ISMRM, Montreal, 2019 #1947.
5) Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015; 33: 550–558.
Figures