2404
Predicting therapeutic response using dynamic enhanced MRI radiomics for patients with HCC treated with TACE: comparison of radiomics models1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Translational Medicine Team, GE Healthcare, Shanghai, China, 3GE Healthcare, Beijing, China
Synopsis
In the current study, dynamic enhanced MRI-based radiomics model was applied to predict therapeutic response in hepatocellular carcinoma treated with transcatheter arterial chemoembolization. In order to obtain the optimal radiomics model, we compared the diagnostic performance of the model built by different classifiers.
Purpose
To investigate the application of dynamic enhanced MRI-based radiomics to predict therapeutic response in hepatocellular carcinoma (HCC) after transcatheter arterial chemoembolization (TACE), and compare the diagnostic performance of the model built by different classifiers.Introduction
HCC ranks fourth as the major cause of cancer-related deaths globally and is the sixth most common cancer in the world. Less than 30% of patients with HCC are eligible for potentially curative therapies, such as resction or transplantation[1, 2]. TACE is a well established therapy for patients with unresectable HCC. It is widely accepted as a means to control tumor growth, to prolong survival in patients with unresectable HCCs, and to decrease the recurrence of resectable HCCs[3, 4]. It is crucial for clinicians to make treatment planning via accurately assessing the therapeutic response before the performance of TACE. Thus, we need to explore an non-invasive method to preoperatively predict therapeutic response of HCC patients before TACE. Radiomics is a rapidly growing field that converts medical images into high-dimensional quantitative features through different algorithms, potentially aidding in tumor diagnosis, pathological grading, treatment response assessment, and prognosis prediction. Therefore, dynamic enhanced MRI-based radiomics was introduced in the present study to evaluate its clinical application performance in predicting therapeutic response of HCC after TACE, and obtain the optimal radiomics model built by different classifiers.Materials and Methods
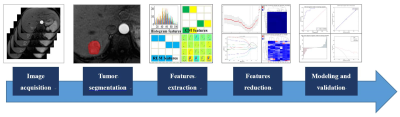
The present study retrospectively enrolled 61 HCCs who were confirmed by biopsy or in accordance with the guidelines of the American Association for the study of liver diseases (AASLD). All patients have underwent preoperative dynamic enhanced MR examinations within 1 month before initial TACE and a follow-up MRI scan after TACE (with 4-8 weeks). The modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria was used to assess the tumor response based on MRI findings, which grades target lesion responses as follows: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). We defined CR and PR as response treatment (RT) group, and SD and PD as non-response treatment (NRT) group. On the arterial phase images of enhanced MR scanning, the radiologist manually outlined the region of interests (ROIs) at each slice of the target lesions by using ITK-SNAP sofaware and extracted 792 radiomics features, which included histogram features, formfactor features, texture (Haralick, GLSZM, GLCM, and RLM) features, and higher order statistics features via Gaussian transformation. In order to reduce features dimensionality, we performed the Spearman analysis and least absolute shrinkage and selection operator (LASSO) algorithm to identify the most predictive radiomics features. The logistic regression (LR), support vector machine (SVM), Bayes, K-nearest neighbor (KNN), and decision tree (DT) classifiers were applied to build the radiomics model for predicting therapeutic response, respectively. The receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic performance. The flow chart of the radiomics processing was shown in Figure 1.Results
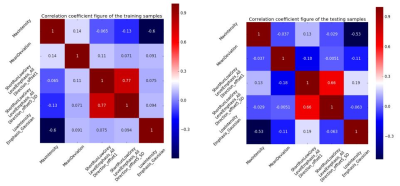
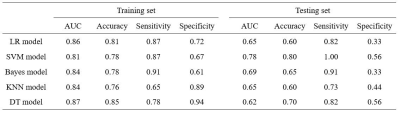
After performing Spearman analysis and LASSO method, there remained five radiomics features which included MaxIntensity, MeanDeviation, ShortRunLowGreyLevelEmphasis_AllDirection_offset1, ShortRunLowGreyLevelEmphasis_AllDirection_offset5_SD and LowIntensityEmphasis_Gaussian. The correlation coefficients of the training and testing samples were shown in Figure 2. The diagnostic performance of the radiomics model that built by five different classifiers were shown in Table 1. The results indicated that SVM classifier on arterial phase images ( AUC: 0.81, accuracy: 0.78, sensitivity: 0.87, specificity: 0.67 in training set; AUC: 0.78, accuracy: 0.80, sensitivity: 1.00, specificity: 0.56 in testing set) was the optimal strategy to identify the effectiveness of TACE for HCC patients.Discussion and Conclusion
Compared with other radiomics models, such as LR, Bayes, KNN and DT models, the radiomics model built by the SVM classifier, showed better performance in predicting treatment response for HCC patients after performing TACE. In the testing set, the AUCs of the LR, SVM, Bayes, KNN and DT models were 0.65, 0.78, 0.69, 0.65 and 0.62, respectively. In conclusion, the arterial phase MR-based radiomics models demonstrated good discriminative ability in predicting therapeutic response in patients of HCC treated with TACE, and SVM model can obtain the optimal diagnostic performance.Acknowledgements
No acknowledgement found.References
[1] Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016, 151: 356–363.
[2] Ding XX, Zhu QG, Zhang SM, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017, 8: 55715–55730.
[3] Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012; 262:708–718.
[4] Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359: 1734–1739.