2396
Radiomics based on multiparametric magnetic resonance imaging to predict extraprostatic extension of prostate cancer1Department of Radiology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China, 2Deepwise AI Lab, Beijing, China, 3Department of Urology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China, 4Department of Pathology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Synopsis
The preoperative prediction of EPE has a profound impact on treatment decision making, however, it still remains challenging presently. In this study, we compared the radiomics signatures extracted from different MR sequences to diagnose EPE. The radiomics signature based on DWI showed better performance for EPE prediction among mpMRI sequences. The radiomics model based on DWI, T2WI and DCE images was demonstrated feasible for the prediction of EPE. But the added value of clinical variables to the radiomics model was not prominent.
Introduction
Prostate cancer (PCa) is the most common malignancy in men worldwide and also the second leading cause of cancer-related death [1]. Studies have shown that the presence of extracapsular extension (EPE) in radical prostatectomy (RP) specimens was highly predictive of death from PCa [3] and indicated a higher risk of biochemical recurrence [4]. The preoperative prediction of EPE has a profound impact on treatment decision making, however, it still remains challenging presently. This study was designed to develop a radiomics model based on multiparametric MRI (mpMRI) for preoperative prediction of EPE in patients with PCa.Methods
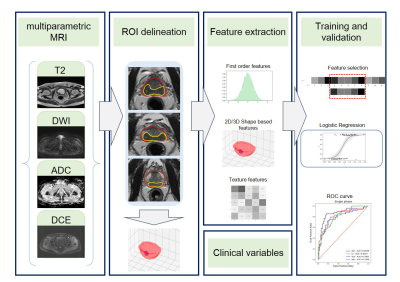
The Institutional Review Board approved this retrospective study and waived the need for written informed consent. This retrospective study enrolled 95 radical prostatectomy-confirmed PCa patients with 115 lesions from January 2015 to March 2019 at our institution. A 3.0-T MRI scanner (GE750, GE Healthcare, Milwaukee, WI) was used to perform prostate mpMRI. Clinical and pathological variables were also obtained. One radiologist (reader A, with 5-year experience in prostate MRI) who was blinded to the pathologic EPE status performed 3D segmentation manually on MR images using Deepwise Research Platform (http://label.deepwise.com) and a senior radiologist (reader B, with 13-year experience in prostate MRI) reviewed all the lesions. The EPE status for each lesion was recorded by a senior pathologist. Radiomics features were extracted from T2-weighted (T2W), diffusion-weighted (DW), apparent diffusion coefficient (ADC) and dynamic contrast-enhanced (DCE) images. A total of 1,231 radiomics features (including first-order statistics, shape features, and texture features) were extracted from the ROI of a single sequence for each lesion. We first randomly split all the samples into two parts, 90% as the training cohort and 10% as the validation cohort, in a stratified manner. Second, after feature standardization and feature reduction, we performed feature selection using F-test for every feature and label pair. Finally, a logistic regression model was applied to select features to build radiomics signatures and a radiomics model (Figure. 1). Repeated stratified 10-fold cross-validation was applied. Next, we took the mean and standard deviation of the area under the curve (AUC) as the performance of our model. The diagnostic performance of the radiomics model was compared with that of the clinical model, and the combined nomogram.Results
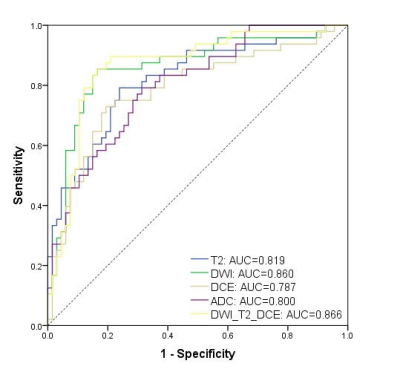
Radiomics signatures based on DWI, T2WI, DCE, and ADC images achieved satisfactory discriminative ability in predicting EPE, with AUC values of 0.902, 0.905, 0.844, and 0.855, respectively, in the training cohort, and 0.860, 0.819, 0.787, and 0.800, respectively, in the validation cohort (Figure. 2). Specifically, in the validation cohort, radiomics features extracted from DWI showed the highest AUC value, with accuracy, sensitivity, and specificity of 81.7%, 85.1%, and 77.1%, respectively. The DWI+T2+DCE model showed fair good results, with AUC, accuracy, sensitivity and specificity values of 0.866, 84.4%, 83.6%, and 85.4%, respectively, in the validation cohort. The radiomics model outperformed the clinical model (AUC=0.780) and was comparable with the combined nomogram (AUC=0.884).Discussion
In this study, we compared the radiomics signatures extracted from different MR sequences to diagnose EPE. Comparatively, in single-sequence analysis, the DWI model achieved a higher AUC value as well as higher accuracy, sensitivity, and specificity than other sequences in the validation cohort. And the combination of DWI, T2, and DCE showed comparable AUC value but higher specificity than using the DWI radiomics signature alone. This radiomics model also showed better diagnostic performance than that of the clinical model. The integration of the clinical model and radiomics model showed similar results to diagnose EPE compared with that using the radiomics model alone.Conclusion
The radiomics signature based on DWI had a better performance for EPE prediction among mpMRI sequences, and the radiomics model based on DWI, T2WI and DCE images might be a feasible tool for predicting EPE, which may assist in the decision-making for individual treatment of PCa. Nevertheless, the additional value of clinical variables to the radiomics model seems not prominent.Acknowledgements
No acknowledgement found.References
1 Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7-30
2 Chen W, Zheng R, Baade PD, et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115-132
3 Bill-Axelson A, Holmberg L, Garmo H, et al (2018) Radical Prostatectomy or Watchful Waiting in Prostate Cancer - 29-Year Follow-up. N Engl J Med 379:2319-2329
4 Jeong BC, Chalfin HJ, Lee SB, et al (2015) The Relationship Between the Extent of Extraprostatic Extension and Survival Following Radical Prostatectomy. Eur Urol 67:342-346