2385
Portable NMR for quantification of breast density and tissue composition: from proof-of-concept in vitro to trials in vivo1School of Chemistry and Physics, Queensland University of Technology, Brisbane, Australia, 2Queensland University of Technology, Brisbane, Australia, 3University of Queensland, Brisbane, Australia, 4Princess Alexandra Hospital, Brisbane, Australia
Synopsis
Elevated mammographic density (MD) is a significant independent risk factor for breast cancer and a source of masking in X-ray mammography. Availability of a low-cost, non-ionising measurement technique for quantitative assessment of MD would be valuable both in therapeutic and research contexts. We demonstrate that Portable NMR, a low-cost technique based on the same physics as MRI, represents a reliable approach to quantification of MD in excised breast tissue samples in vitro. We also present the initial results of an observational trial in healthy volunteers, which show that Portable NMR has a great potential for quantification of MD in vivo.
INTRODUCTION
Elevated mammographic density (MD) is an independent risk factor for breast cancer (BC) and a source of masking in X-ray mammography. It is hypothesised that high-frequency longitudinal monitoring of MD can be beneficial in hormonal BC prevention, where early MD changes (or lack thereof) herald the eventual success or failure of the treatment. Such measurements would also be valuable for monitoring the transient effects of lifestyle or the menstrual cycle on MD, which would enable insights into the fundamental biology of MD and its relationship to the individual’s hormonal status.Single-sided portable NMR is a low-cost measurement technique that is based on the same fundamental physics as MRI. It has been widely used for profiling of materials in industrial and manufacturing settings, and over the last several years the technique has experienced significant growth in the number of biomedical applications. We demonstrate that portable NMR represents a novel approach to quantification of MD and breast tissue composition. Its advantages in the context of MD measurement are the low cost of portable-NMR instrumentation, suitability for measurements in vivo, and the absence of ionising radiation.
METHODS
We have investigated the utility of Portable NMR for quantitative characterisation of MD both in excised breast tissue samples in vitro and in healthy volunteers in vivo. Excised breast tissue samples were obtained from prophylactic mastectomy or breast-reduction procedures. Three measurement methods were examined in vitro: (1) saturation-recovery (T1) with monoexponential analysis; (2) Carr-Purcell-Meiboom-Gill (CPMG) decays (T2) with either inverse Laplace transform (ILT) or monoexponential analysis; and (3) stimulated-echo diffusion measurements with biexponential analysis. X-ray mammography and Computed Tomography (micro-CT) measurements were used as “gold standards” for the excised sample measurements.Healthy volunteers were recruited for an observational trial with the sole purpose of ascertaining the feasibility portable-NMR MD profiling in vivo. The portable-NMR depth profiles of the patients’ mammographic density were measured in the upper outer quadrant of the right breast in 2 mm depth steps over the depth range from 0 to 21 mm. At each depth, the relative amounts of fibroglandular (FGT) and adipose tissue were measured from the inverse Laplace transforms of the respective CPMG decay. The gold standard was the depth profile of FGT and adipose tissue reconstructed from a set of 3D MRI inversion recovery-prepared FLASH images with the inversion recovery times of 210 ms, 690 ms and 3000 ms.
RESULTS
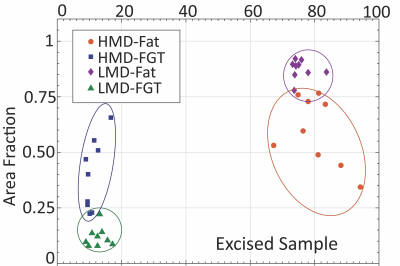
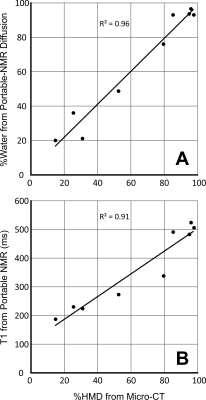
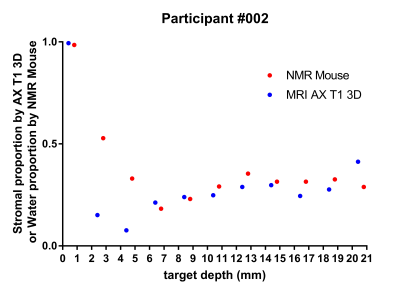
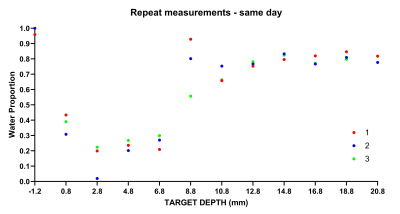
All measurements performed in vitro (T1, T2 and diffusion) were able to reliably classify regions of High and Low mammographic density selected by the radiologist (T2-based results are shown in Fig. 1). When MD was assessed on a continuous scale, longitudinal spin-relaxation time constants (T1) and the relative tissue water content obtained from portable-NMR diffusion measurements exhibited an excellent correlation with the gold standard (micro-CT) (Fig. 2). Both T2 and diffusion measurements enabled quantification of the Fat:Water ratio within the samples. This ratio agreed well with the FGT:adipose tissue ratio within the samples obtained using micro-CT images.A comprehensive analysis of the observational trial data is still underway. Among the data already analysed, the depth profiles of the relative content of Water and Fat obtained from ILT T2 analysis are in good agreement with the results of quantitative 3T MRI measurements (Fig. 3). Repeated same-day measurements of a single individual demonstrated good reproducibility (Fig. 4).
DISCUSSION
Clinical MRI has a well-demonstrated capability to provide the 3D maps of MD-equivalent quantities with excellent spatial resolution and an excellent agreement with traditional mammography. However, cost is a major barrier to the adaptation of MRI for routine monitoring of MD. As a low-cost technique, Portable NMR overcomes this barrier while also avoiding the use of ionising radiation for breast imaging. Therefore, Portable NMR is promising as a means of monitoring MD in human subjects, and following a comprehensive validation it could either complement or serve as an alternative to standard X-ray-based mammography.Our in vitro results demonstrate that Portable NMR is a reliable technique for quantification of MD in excised breast tissue samples. The initial results of the measurements performed in vivo are also encouraging, although a comprehensive analysis of the data is still in progress.
CONCLUSIONS
Systematic trials to evaluate the accuracy and reproducibility of Portable NMR-based quantification of MD in vivo are warranted. The ability to quantify tissue composition, combined with low cost of instrumentation, already make Portable NMR a promising modality for clinical mammographic imaging. More broadly, Portable NMR represents an emerging new paradigm in quantitative characterisation of soft tissues.Acknowledgements
We thank Dr Andrew Coy and Dr Robin Dykstra (Magritek Ltd) and A/Prof Petrik Galvosas (Victoria University Wellington, New Zealand) for the loan of PM5 NMR-MOUSE instrument. This work was funded by the Princess Alexandra Hospital Research Foundation and Translational Research Institute.References
1. M.C. Tourell, T.S. Ali, H.J. Hugo, et al, T1-based sensing of mammographic density using single-sided portable NMR. Magn. Reson. Med. 2018, 80, 1243–1251.
2. T.S. Ali, M.C. Tourell, H.J. Hugo, et al, Transverse relaxation-based assessment of mammographic density and breast tissue composition by single-sided portable NMR. Magn. Reson. Med. 2019, 82, 1199–1213.
3. X. Huang, T.S. Ali, T. Nano, et al, Quantification of breast tissue density: Correlation between single-sided portable NMR and micro-CT measurements. Magn. Reson. Imaging 2019, 62, 111–120.
Figures