2384
A feasibility study of radiomics based on dynamic contrast enhanced magnetic resonance imaging in identifying benign and malignant breast mass.1The second affiliated hospital of xi 'an jiaotong university, xi an, China
Synopsis
The current study aim to investigate the value of radiomics’ features based on dynamiccontrast-enhanced magnetic resonance imaging (DCE-MRI) in the identification of benign andmalignant breast tumors. It was concluded that the radiomics’ features based on dynamicenhanced magnetic resonance imaging have certain value in identifying benign and malignantbreast tumors, among them, the superior radiomics’ features is Gabor, gray level co-occurrencematrix variance and variance, which worth further study.
Introduction
As breast cancer is the common malignant tumor in the world[1], obtaining an accurate diagnosis is significant for it contributes to the treatment and the prognostic outcomes of the disease. MRI plays an important role in the early and precise diagnosis of breast disease.The traditional breast MRI parameters based on tumor morphology, internal reinforcement and hemodynamic characteristics are poorly specificity and may be overlap the benign and malignant tumors, which can lead to overtreatment[2]. Biopsy is invasive and can’t assess comprehensively for the heterogenous breast cancer. However, radiomics can reflect the space-time heterogeneity of tumors without invasive by extracting and analyzing a large number of high-dimensional and quantitative image features from X-ray, CT, MRI, PET, US and other medical images[3].The purpose of this study is to preliminarily investigate the performance of radiomics’ features based on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in identifying benign and malignant breast tumors.Material and Methods
From Dec 2015 to Jul 2018, 110 patients(all females, 15~60 years old,mean 39.90 years)in our hospital both with solitary breast masses confirmed by pathology and receive a baseline MR examination prior to any treatment were retrospectively included. All of the patients were randomly assigned to the training set and validation set. Two radiologists, independently based on the baseline DCE-MRI, selected regions of interest(ROI) including the largest area of the breast mass layer by layer(Figure1.1,1.2). Then extracted total of 338 radiomics’ features from the ROI by Matlab software (Matlab2016a,Table 1). All of the masses builded the patch scheme to extract characteristics, so that the sample patch level used in the training model is close to 180,000, which greatly compensates for the smaller sample size and that the model could be fully trained. Then random forest classification model was constructed to predict the benign and malignant breast masses. The MR imaging was performed using a 3.0 T system (Signa HDxt; GE Medical Systems, Milwaukee, WI, U.S.) equipped with 8-channel breast coils in prone position of patients. The DCE-MRI were taken at 64 s, 128 s, 192 s, 256 s, and 318 s after the injection of 0.1 mmol/kg body weight of gadolinium-DTPA (Magnevist, Schering, Germany) at a rate of 2.0 ml/s followed by 20 ml saline solution. T1-weighted fast spoiled gradient-recalled echo sequence with parallel imaging (VIBRANT) sequence on the transverse plane (TR/TE/TI = 4.4/2.1/125 ms, flip angle 14o, matrices = 416×320, section thickness/spacing = 1.6/0 mm, and field of view= 350×350 mm) were used. Statistical analysis applied SPSS19.0 software. The measurement data is described in the median.The receiver operating characteristic (ROC) evaluates the diagnostic capability of the identification model.Results
There are totally 40 benign lesions and 70 malignant lesions in the study. The benign including 14 fibromyaloma, 9 cystic hyperplasia, 2 papilloma, 8 breast hyperplasia, 2 duct ectasia and 5 cases of granuloma or purulent inflammation,while the malignant including 62 non-special type of invasive carcinoma, 1 small tube carcinoma, 3myelin carcinoma, 2 mucus carcinoma, 1sieve carcinoma and 1 glycogen-rich clear cell carcinoma. The training set is 89 cases (57 malignant, 32 benign) and the validation set is21 cases(13 malignant, 8 benign). As a result, the more significant radiomics’ features are Gabor, grayscale symbiotic matrix variation (GLCM-difference variance) and variance. The radiomics models diagnostic efficacy of identifying malignant and benign breast lesions based on DCE-MRI is shown in Table 2 and Figure 2.1 and 2.2. The AUC, accuracy, sensitivity, specificity, and positive predictions were above 0.90 in the training set, with AUC value and positive prediction up to 0.98 and negative prediction of 0.81. In the validation set, the AUC, accuracy, sensitivity, specificity and positive prediction up to 0.90 or above, with positive prediction of 0.97 and negative prediction value of 0.76.Discussion and Conclusion
Main finding in our study is that the diagnostic efficacy of radiomics based on DCE-MRI is higher than that of dynamic signal strength (AUC 0.72-0.88) [4]. We established the verification set, whose AUC, accuracy, sensitivity, specificity and positive prediction value up to more than 0.90, indicating that the identification model established in our study stabilitily, while there are few studies at home and abroad to establish validation set. The results of this study are similar to those of Pickles and his colleagues[5] who combined with texture dynamic characteristics (AUC 0.94, accuracy 0.90, sensitivity 0.92), however our study with higher specificity of 0.91 (0.85). It is possible that the sample size in our study was increasing(it was malignant 30, benign 22), the pathological types were abundant, and the manual image segmentation. We established a random forest classification model, which is similar to the study of Bickelhaupt[6]. The major limitations in our study are all lesions were single-sided masses, without including the two-sided, one-sided multiple lesions or non-mass lesions. The number of subjects is small and a single-center study. Hence future study with more subjects of multi-center is warranted. To conclude, the radiomics’ features based on DCE-MRI have certain value in identifying benign and malignant breast tumors.Acknowledgements
No acknowledgement found.References
[1] Freddie B, Jacques F, Isabelle S, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries [J].CA Cancer J Clin, 2018, 68(6):394-424.
[2] Byers T, Wender RC, Jemal A, et al. The American Cancer Society challenge goal to reduce US cancer mortality by 50% between 1990 and 2015: Results and reflections [J].CA Cancer J Clin, 2016, 66(5):359-369.
[3] Santamaría G, Bargalló X, Fernández PL, et al. Neoadjuvant Systemic Therapy in Breast Cancer: Association of Contrast-enhanced MR Imaging Findings, Diffusion-weighted Imaging Findings, and Tumor Subtype with Tumor Response. Radiology. 2017 Jun;283(3):663-672.
[4]Khouli RH, Macura KJ, Barker PB, et al. Relationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breast[J].JMRI. 30(5):999-1004.
[5]Pickles MD, Lowry M,Gibbs P.Pretreatment Prognostic Value of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Vascular,Texture,Shape, and Size Parameters Compared With Traditional Survival Indicators Obtained From Locally Advanced Breast Cancer Patients[J]. Invest Radiol, 2016, 51(3): 177-85.[6]Bickelhaupt S, Jaeger PF, Laun FB, et al. Radiomics based on adapted diffusion kurtosis imaging helps to clarify most mammographic findings suspicious for cancer [J]. Radiology 2018, 287(3):761-770.
Figures
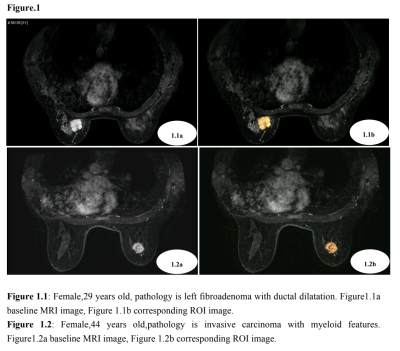
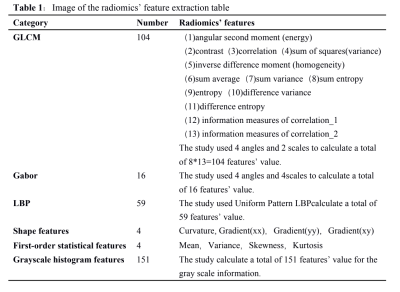
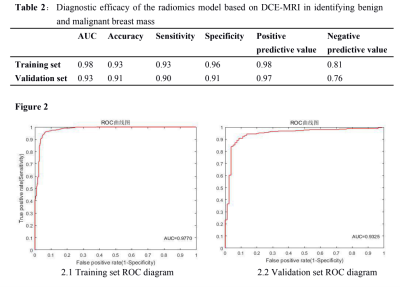
Table 2:Diagnostic efficacy of the radiomics model based on DCE-MRI in identifying benignand malignant breast mass
Figure 2.1 Training set ROC diagram Figure2.2 Validation set ROC diagram