2379
Use of a Digital Reference Object for Validation of Advanced Reconstruction Methods in High Spatial-temporal Resolution Breast DCE-MRI
Ping N Wang1, Julia V Velikina1, Roberta M Strigel1,2,3, Leah C Henze Bancroft2, Ty A Cashen4, Kevin M Johnson1, Alexey A Samsonov2, Ali Ersoz5, Edward F Jackson1,2,3, and James H Holmes2
1Department of Medical Physics, University of Wisconsin Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin Madison, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 5MR Engineering, GE Healthcare, Waukesha, WI, United States
1Department of Medical Physics, University of Wisconsin Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin Madison, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 5MR Engineering, GE Healthcare, Waukesha, WI, United States
Synopsis
Advanced data acquisition and reconstruction methods have been proposed to improve temporal and spatial resolution DCE imaging for breast. However, validation and comparison of these methods against a known truth is challenging. In this work we propose a digital reference object for breast pharmacokinetic simulation to evaluate different advanced reconstructions including MOCCO and iGRASP. The approach allowed comparison of the reconstructed temporal characteristics including pharmacokinetic analysis using different reconstruction parameters against the assigned ground truth. Spatial sharpness was also measured to compare with reference fully sampled images.
Introduction
Breast dynamic contrast enhanced MRI (DCE-MRI) is widely accepted as the most sensitive modality with moderate specificity for detection of breast cancer1. Quantitative pharmacokinetic (PK) modeling of DCE-MRI has been proposed to improve the specificity2. However high temporal resolution is required for accurate estimation of the PK parameters while high spatial resolution is needed to detect and characterize lesions3. Compressed sensing (CS) reconstructions have shown promise to improve temporal resolution, however it is often necessary to optimize reconstruction parameters for application or even subject specific imaging scenarios4. Two promising reconstruction approaches include iGRASP, which relies on a total variation (TV) sparsifying transform5, and MOCCO6, which uses temporal models estimated from the imaging data to reduce the sensitivity to modeling error. Even though these advanced algorithms achieved remarkable spatial recovery with high undersampling factors, the accuracy of the temporal curves remains an open question due to the lack of ground truth in patients. Digital reference objects (DROs) provide a platform to validate the temporal accuracy of different acquisition and reconstruction parameters7,8 in the presence of simulated signal changes such as those provided by PK modeling. In this study, we demonstrate the use of a breast PK simulation DRO for investigating the performance of advanced reconstruction methods including SENSE, MOCCO, and iGRASP.Theory
Time-resolved images were reconstructed using iGRASP and a modified MOCCO algorithm6. iGRASP reconstructions were performed using publicly available code provided by Feng et al5,9 including temporal TV regularization. The underlying temporal model for MOCCO was obtained from the low frequency region from fully-sampled central k-space data using progressive learning with cubic spline approximation10,11 followed by complex independent component analysis12.Methods
DRO Simulations: Simulation 1 was performed to benchmark performance of the DRO13 with iGRASP using the published acquisition and reconstruction parameters from Kim et al.9 including temporal regularization parameters of λ= 0.5 and 2. This produced a 5-s temporal resolution (undersampling factor R=12). Simulation 2 was modeled after our local clinical axial protocol (TR/TE= 5.5/2.4 ms, FOV= 340 x 340 mm, 448x448 in-plane matrix, 142 z-phase encodes, 1.5x out-of-plane acceleration). The simulated radial data were reconstructed at 10-s temporal resolution, corresponding to a higher acceleration of R=44 (16 projections/frame). Values of 0.5 and 10 were chosen for λ in iGRASP and MOCCO respectively to optimize spatial and temporal resolution. An idealized fully sampled dataset using SENSE reconstruction was generated at 10-s temporal resolution as a reference. Temporal curves from regions-of-interest placed in each lesion were also measured using normalized root-mean-square error (nRMSE). PK model fitting was performed using extended Tofts model13 to assess the ability to recover the initial contrast kinetic parameters from the reconstructed data. In-vivo Assessment: A patient volunteer was imaged during contrast injection (gadobenate dimeglumine, Multihance; Bracco Inc, Milan, Italy) on a clinical 3T MRI (Signa Premier, GE Healthcare, Waukesha, WI) using a 16-channel breast coil (Sentinelle, Invivo International, Gainsville, FL) for this IRB-approved, HIPPA-compliant study. A 3D radial stack-of-stars gradient echo sequence was used to collect 1344 unique radial projections and reconstructed using MOCCO at 10-s temporal resolution.Results
Simulation1: Qualitative assessment found the choice of λ=0.5 for iGRASP provided the best compromise between spatial quality and temporal recovery in the DRO (Fig. 1), resulting in uptake slopes that were well-matched to the ground truth. Loss of temporal fidelity was observed at higher values (λ=2).Simulation2: iGRASP with λ=0.5 resulted in high spatial resolution but did retain some undersamplling artifacts (Fig2 c). Increasing λ was found to reduce these undersampling artifacts but compromise temporal fidelity (results not shown). iGRASP with λ=0.5 performed well for recovering ktrans, which is consistent with the well depicted wash-in slope (Fig 3). MOCCO using λ=10 showed overall similar image quality (Fig2 b) compared to reference images (Fig2 a). The temporal curves were well recovered by MOCCO reconstruction with nRMSE less than 10% (Fig 3), allowing good recovery of Ve. Simulated lesions with the most rapid wash-in and wash-out slopes (lesions 3 and 5) were difficult to recover for both techniques. iGRASP with λ=0.5 was found to yield some temporal blurring in these regions of fast kinetics leading to fitting errors in Ktrans, Ve, and Vp (Fig. 4). MOCCO showed better recovery of the overall curve shapes but with slight underestimation of the peak intensity resulting in limited errors in Ve and Vp. In vivo results are shown for a patient volunteer using the optimized reconstruction parameters based on simulation2.
Discussion and conclusions
We demonstrated the feasibility of using a DRO breast phantom to optimize and evaluate the performance of advanced reconstruction techniques for recovering temporal kinetics in breast DCE-MRI. As a publically available technique, iGRASP provides an accessible starting point for accelerated imaging applications. Analysis found that λ=0.5 resulted in the optimal balance of temporal and spatial accuracy including capturing the wash-in slope, which is consistent with prior work looking at use in abbreviated breast protocols14. Evaluation of the second method, MOCCO, showed high image quality with minimal loss of temporal fidelity, indicating that it may be well suited to PK modeling or treatment monitoring applications. Future work will look at evaluating the predictions of these DRO simulations when applied to in vivo and disease settings.Acknowledgements
The authors wish to acknowledge support from NIH-R21EB018483, NIH-R01EB027087, GE Healthcare, the RSNA Research and Education Foundation, and a Research and Development Grant from the Departments of Radiology and Medical Physics, University of Wisconsin.References
- Kuhl, C. K. et al. Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer. J. Clin. Oncol. 23, 8469–8476 (2005).
- Furman-Haran, E., Schechtman, E., Kelcz, F., Kirshenbaum, K. & Degani, H. Magnetic resonance imaging reveals functional diversity of the vasculature in benign and malignant breast lesions. Cancer 104, 708–718 (2005).
- Henderson, E., Rutt, B. K. & Lee, T.-Y. Temporal sampling requirements for the tracer kinetics modeling of breast disease. Magn. Reson. Imaging 16, 1057–1073 (1998).
- Wong, A., Mishra, A., Fieguth, P. & Clausi, D. A. Sparse Reconstruction of Breast MRI Using Homotopic L1 Minimization in a Regional Sparsified Domain. IEEE Trans. Biomed. Eng. 60, 743–752 (2013).
- Feng, L. et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI: iGRASP: Iterative Golden-Angle RAdial Sparse Parallel MRI. Magn. Reson. Med. 72, 707–717 (2014).
- Velikina, J. V. & Samsonov, A. A. Reconstruction of Dynamic Image Series from Undersampled MRI Data Using Data Driven Model Consistency Condition (MOCCO). 12.
- Wang, P. et al. Feasibility of High Spatial and Temporal Resolution Breast DCE-MRI using Radial Acquisition with Data-Driven Model Consistency Condition Reconstruction. in. Proceedings of the 26th ISMRM Scientific Meeting 2018 (2018)
- Wang, P. et al. Breast DCE-MRI using Radial Acquisition with Data-Driven Model Consistency Condition Reconstruction. in. Proceedings of the 27th ISMRM Scientific Meeting 2019 (2019)
- Kim, S. G. et al. Influence of temporal regularization and radial undersampling factor on compressed sensing reconstruction in dynamic contrast enhanced MRI of the breast: Temporal Regularization and Radial Undersampling Effects on DCE-MRI. J. Magn. Reson. Imaging 43, 261–269 (2016).
- Velikina, J. et al. Ultrafast Speech Imaging at High Spatial Resolution using Model-Consistency Condition Reconstruction with Progressive Temporal Basis Learning. Proc. 26th ISMRM Sci. Meet. 2018 (2018).
- Boor, C. de. A Practical Guide to Splines. in Applied Mathematical Sciences (1978). doi:10.1007/978-1-4612-6333-3.
- Novey, M. & Adali, T. On Extending the Complex FastICA Algorithm to Noncircular Sources. IEEE Trans. Signal Process. 56, 2148–2154 (2008).
- Henze, L. Digital Breast Phantom for Evaluating Dynamic Accelerated Imaging Methods. in Proceedings of the 18th ISMRM Scientific Meeting 2010 (2010).
- Tofts, P. S. et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. 10.
- Sadinski, M. et al. Sparse Radial VIBRANT: High Spatiotemporal Resolution Dynamic Contrast Enhanced MRI with Compressed Sensing in Breast. in. Proceedings of the 27th ISMRM Scientific Meeting 2019 (2019)
Figures
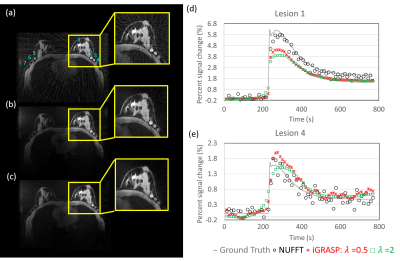
Figure1: Results
from simulation 1. The
DRO phantom was used to generate radial data for a reconstructed time
series consisting of images with a matrix size of 256x256 and 5-second
temporal resolution. The simulated radial data was reconstructed using (a)
conventional gridding (NUFFT), (b) iGRASP using λ=0.5 and (c) iGRASP using λ=2.
Significant reduction of streaking artifacts was shown with increasing the λ value but (d,e) the lesion curves showed
temporal blurring as a tradeoff. Note the curves were re-scaled to match the
ground truth intensity due to the scaling effect in the NUFFT.
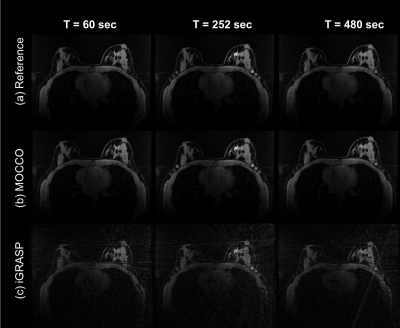
Figure2: Representative
time frames comparing the fully-sampled reference time series with undersmapled
reconstructions using MOCCO and iGRASP at 10s temporal resolution and an
imaging matrix of 448x448 for Simulation 2. With
R=44, residual streaking artifacts were visible in the fibroglandular tissue
and axilla region using iGRASP with the λ=0.5,
which provided better temporal recovery.
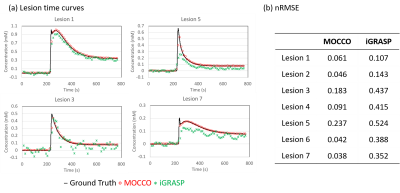
Figure3: Representative
tissue concentration time curves from four lesions with different PK parameters
from Simulation 2. (a) Curves from iGRASP show slight oscillations due to
presence of undersampling artifacts in both pre-contrast and wash-out frames,
corresponding to lower nRMSE in (b). Curves of lesion 3 and 5 showed the
largest nRMSE and were underestimated by both reconstruction methods due to the
sharp wash-in and wash-out slope.
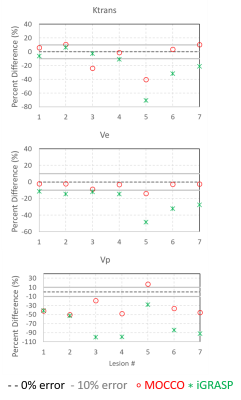
Figure4: PK
parameters fit from MOCCO and iGRASP both reconstructed at 10s temporal
resolution from Simulation 2. Both MOCCO and iGRASP showed promising results in
Ktrans and Ve (within 10% error range). However, underestimation was observed
for both methods in lesions demonstrating rapid temporal enhancement (lesion
5). MOCCO also showed underestimation of
ktrans in lesions (lesion 3), while iGRASP
showed underestimation of ktrans and Ve in lesions located in low SNR regions
(lesions 5-7).
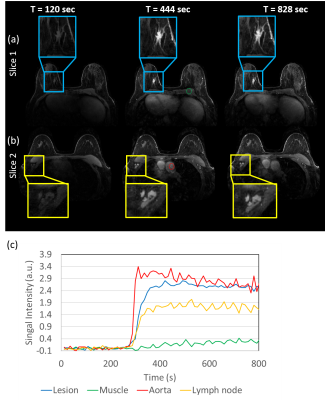
Figure5: Time-resolved
DCE images from a patient volunteer reconstructed using MOCCO with 10-s
temporal resolution. High image quality is observed along all time frames. Concentration
time curves are plotted from ROIs placed in the heart (red), lesion (blue),
lymph node (yellow) and muscle (green). Rapid wash-in and wash-out contrast
kinetics are observed in the heart. The lesion showed relatively rapid contrast
uptake (blue), while the other two tissues (yellow and green) showed slower
contrast uptake.