2366
Investigation of Lung Injury Caused by Air pollutants PM2.5 with both Pulmonary Function Tests and Hyperpolarized 129Xe MR1National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy of Precision Measurement Science and Technology, Chinese Academy of Sciences-Wuhan National Laboratory for Optoelectronics, Wuhan, China, 2Department of Radiology, Chinese PLA General Hospital, Beijing, China
Synopsis
In this study, hyperpolarized 129Xe MR and pulmonary function tests were utilized to quantitatively evaluate the pulmonary physiological changes caused by air pollutants (PM2.5), which are difficult to be non-invasively assessed using the conventional methods including bronchoalveolar lavage fluid analysis and histopathological sections. Significant differences were found in mean exchange time constant, septal wall thickness and TP/GAS ratio using 129Xe dynamic spectra. The results from our study indicated hyperpolarized 129Xe MR may be a promising method for quantifying lung injury caused by air pollution in clinic.
Introduction
As a major health burden for countries such as China, outdoor air pollution, especially the ambient fine particulate matter (PM2.5) has contributed to the increased incidence, prevalence and mortality of lung disease 4,5. Bronchoalveolar lavage fluid analysis and enzyme-linked immunosorbent assay are the widely used methods for evaluating the lung injury caused by air pollutants (PM2.5) in the previous studies 3-5, However, these methods are invasive and are not able to quantify the gas exchange function of the lung. Hyperpolarized (HP) 129Xe MR is a promising method in quantifying the pulmonary microstructure and physiological function in vivo, and it has been widely utilized in non-invasively assessing pulmonary diseases such as COPD, IPF, and asthma 6-8. In this study, HP 129Xe MR and pulmonary function tests (PFTs) were used to evaluate pulmonary function changes caused by air pollutants (PM2.5) in animals.Methods
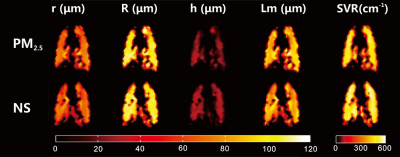
PM2.5 was collected using an air particle sampler (TH-1000H; Wuhan Tianhong, China) and prepared as the solution 3,5. A total of 12 Sprague-Dawley rats were divided into two groups. The experimental group was intratracheally instilled with PM2.5 solution (16.2 mg/kg body weight, twice a week) for four weeks, while the control group treated with an equivalent amount of normal saline. Seven days after treatment, the PFTs, HP 129Xe chemical shift saturation recovery (CSSR) spectroscopy and multi-b-value diffusion-weighted imaging (DWI) were performed on all rats. PFTs were performed on a Forced Maneuvers system (CRFM 100; EMMS, UK) with rats breathed air spontaneously. HP 129Xe experiments were performed on 7.0 T animal MRI scanner (Bruker Biospec 70/20 USR; Germany), and the rats were ventilated using a home-built hyperpolarized gas delivery system. For CSSR experiments, 24 exchange time points ranging from 2 ~ 400 ms were used, and xenon signal in tissue and plasma (TP) and red blood cell (RBC) were normalized by the actual xenon signal in the alveoli, and then fitted to model of xenon exchange 9 (MOXE) to obtain the lung physiological parameters including mean exchange time(T) and septal wall thickness (d). For 129Xe DWI experiments, images were acquired using 2D FLASH with the following parameters: FOV=6 cm, matrix=64×64, α=10°, TE=3.52 ms, ramp up/down time=0.123 ms, constant time=1.3 ms, diffusion time=0.7 ms, eight b value (4, 8, 12, 16, 20, 24, 28 and 32 s/cm2). The 129Xe DWI images were fitted to the cylindrical geometrical model 10 to obtain the microstructural parameters such as external radius (R), internal radius (r). After the MR experiments, quantitative histology was performed on H&E stained lung sections to measure the septal thickness.Results
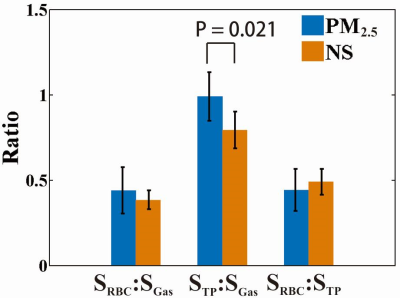
The mean value of RBC/GAS, TP/GAS, and RBC/TPA at the exchange time of 100 ms were shown in figure 1. Among all rats, the mean TP/GAS ratio readily separated the experimental rats and control rats (1.019 ± 0.140 vs. 0.828 ± 0.115, p = 0.021). Similarly, according to results from MOXE fitting, the mean exchange time (T) and septal wall thickness (d) were significantly different between the experimental group versus the control group (14.00 ± 2.84 ms vs. 11.74 ± 2.39 ms, p < 0.05 and 6.74 ± 0.52 μm vs. 6.17 ± 0.48 μm, p < 0.05). Moreover, the septal thickness derived from quantitative histology (6.20 ± 0.36 μm and 5.52 ± 0.32 μm for experimental and control rats, respectively. p < 0.05) correlated well with that derived from CSSR (R2 = 0.8). In 129Xe DWI, the maps of lung microstructural parameters are similar and homogeneous in both groups, as shown in figure 2. No significant difference was shown in the results of PFTs.Discussion and Conclusion
In this study, we demonstrated the feasibility of pulmonary function tests and hyperpolarized (HP) 129Xe MR in evaluating the lung injury caused by air pollutants. The significant increase of exchange time constant and septal wall thickness in experimental rats were most possibly caused by the exudation and infiltration of polymorphonuclear neutrophils in alveoli, consistent with the previous study.3,4 No significant difference was found in the results of PFTs and 129Xe DWI, and the most possible reason is the stage of animal model in this study was too early, and the pulmonary physiological changes cannot be detected by PFTs and 129Xe DWI. Our results indicated HP 129Xe MR is a potential method for quantitatively evaluating the pulmonary injury caused by air pollutants in clinic in the future.Acknowledgements
This work was supported by National Key R&D Program of China (2018YFA0704000), National Natural Science Foundation of China (81625011, 91859206, 21921004, 81601491), Key Research Program of Frontier Sciences, CAS (QYZDY-SSW-SLH018) and Hubei Provincial Natural Science Foundation of China (2017CFA013, 2018ACA143).References
1. Chen Z, Wang JN, Ma GX, Zhang YS. China tackles the health effects of air pollution. Lancet 2013; 382: 1959-1960.
2. Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 2016; 388: 1939-1951.
3. Zhang SY, Shao D, Liu H, et al. Metabolomics analysis reveals that benzo[a]pyrene, a component of PM (2.5), promotes pulmonary injury by modifying lipid metabolism in a phospholipase A2-dependent manner in vivo and in vitro. Redox Biol 2017; 13: 459-469.
4. Kong H, Xia K, Pan L, et al. Autophagy and lysosomal dysfunction: A new insight into mechanism of synergistic pulmonary toxicity of carbon black-metal ions co-exposure. Carbon 2017; 111: 322-333.
5. Zhang Y, Hu H, Shi Y, et al. H-1 NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Sci Total Environ 2017; 589: 212-221.
6. Qing K, Mugler JP, III, Altes TA, et al. Assessment of lung function in asthma and COPD using hyperpolarized Xe-129 chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR Biomed 2014; 27: 1490-1501.
7. Stewart NJ, Leung G, Norquay G, et al. Experimental validation of the hyperpolarized Xe-129 chemical shift saturation recovery technique in healthy volunteers and subjects with interstitial lung disease. Magn Reson Med 2015; 74: 196-207.
8. Li H, Zhang Z, Zhao X, et al. Quantitative evaluation of radiation-induced lung injury with hyperpolarized xenon magnetic resonance. Magn Reson Med 2016; 76: 408-416.
9. Chang YV. MOXE: a model of gas exchange for hyperpolarized Xe-129 magnetic resonance of the lung. Magn Reson Med 2013; 69: 884-890.
10. Sukstanskii AL, Yablonskiy DA. Lung morphometry with hyperpolarized Xe-129: theoretical background. Magn Reson Med 2012; 67: 856-866.