2362
Reducing the breath hold time required to image the diaphragm in an upright position1Physics, University of Nottingham, Nottingham, United Kingdom, 2Medicine, University of Nottingham, Nottingham, United Kingdom
Synopsis
We investigated accelerating two paradigms of in vivo diaphragm imaging using a 0.5T upright scanner. These two paradigms, high resolution and low resolution, were optimised to deliver minimal artefacts and maximal information about the diaphragm surface. These scans delivered sufficient image quality for the tissue boundary of the diaphragm to be located. This will allow for future work to study the diaphragm in the upright position of participants with COPD who are only able to hold their breath for ~5 sec.
Introduction
Disorders of the diaphragm can have serious effects on health, but people with serious respiratory conditions such as COPD can have severe problems lying supine. Therefore it is necessary to study diaphragmatic function in a seated position limiting the use of conventional MRI3. Our Paramed 0.5T MR Open scanner allows for participant positions including, supine and sitting. However, the permanent magnet design and reduced gradient performance necessitated by the open geometry, causes scan times to be longer than on conventional scanners. This can limit the use of breath-hold imaging particular in patients with respiratory diseases (for instance in severely incapacitated participants with COPD, the breath-hold time is restricted to a few seconds). In order to reduce the scan time to acceptable levels the field of view or image resolution may be reduced, however, this prevents the resolution of important structures due to wrap-around artefacts or poor PSF. Here we have implemented compressed sensing on an open MRI scanner to access short imaging times for imaging respiratory function in short breath-holds.Method
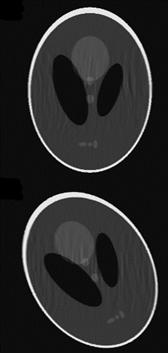
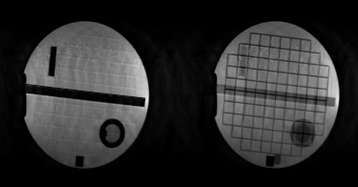
In compressed sensing, only a subset of the phase encode lines are sampled. In order to reconstruct the missing points, assumptions are made about the MRI images1. Initially, a 3D GRE sequence (256x200x22) was targeted with a TR/TE=15.4/8msec in order to image the diaphragm. This scan would take approximately 68sec, which is not possible within a breath-hold. An accel factor of 3 and a fully-sampled scan in the same time were acquired (~22 sec), as well as a highly accelerated sequence at a lower resolution suitable for COPD patients with severally reduced breathing capacity (5.1 sec). Small datasets such as these are typically less sparse, and so the maximum acceleration factors cannot be as high as for larger datasets. Digital phantoms were used to design a sampling pattern, these have different sparsity than the target anatomy (fig1). The sampling pattern was optimised to remove ghosting artefacts, retain tissue boundaries, and to maximise SSIM and VIF. Variable density, elliptical Poisson disk sampling was used, with additional points added-in uniformly, at random. This sampling pattern was then checked by retrospectively undersampling fully sampled data taken of the lung and diaphragm. The reconstruction was performed in BART2. Three regularises were used: L1 sparsity in image and wavelet space, and TV. The regularisation level was optimised for each dataset. The required sampling patterns were implemented on the scanner via the underlying TechMag Redstone spectrometer. Phantom and in vivo undersampled data was then acquired with 5 dummy lines before each scan to allow the magnetization to reach a steady-state.Results
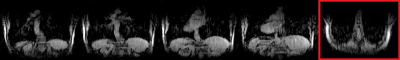
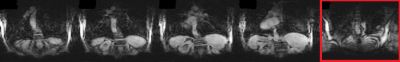
Fig2 shows results of the sampling and reconstruction applied to an ACR phantom. Some ‘noise-like’ artefacts are present, but key resolution is retained. This scan takes 22.6sec, which is within a breath-hold for healthy volunteers. This sequence was then applied in vivo on a healthy volunteer (Fig3). Fig4 shows results from a fully sampled scan acquired in the same time. The boundary of the diaphragm is more clearly defined in the accelerated image, without the wrapping artefacts present in some slices of the fully sampled image (an affected slice is outlined in red). Fig5 shows the highly accelerated scan acquired in 5sec, at lower resolution and with fewer slices. Key tissue boundaries are still preserved, and could still be automatically detected (panel in red).Discussion
This initial work demonstrates the usefulness of the MR Open system with compressed sensing for diaphragm imaging, even in cases where participants are not able to complete a typical length breath-hold. The ability to optimise the sampling and reconstruction process in order to extract the key information from an acquisition is a useful tool for future research. With few Kz points the likelihood of ghosting in this direction is higher, the artefacts created by the sampling are not ‘noise-like’, and so are not removed by the compressed sensing reconstruction. However, the alternative is either increasing the voxel size, or reducing the field of view. The optimal choice depends on the specific case, however, in this scenario the fold-over artefacts in the fully sampled scan obscure anatomy. Several methods exist for accelerating MRI scans, however, the receive coil available only has one channel preventing the use of SENSE/GRAPPA acceleration.Conclusion
In future work, the sampling will be further optimized for the definition of the surface of the diaphragm in the images, and k-space filters will be used to correct the approach to steady-state instead of using dummy scans. We intend to image COPD participants in order to measure properties of the diaphragm, in order to understand the effects of respiratory conditions in specific individuals, where further acceleration may be required individually. In addition, to better quantify the respiratory mechanics, we will be able to image at different points in the breathing cycle and in different positions. Similar acceleration methods could also be applied to studies involving participants who may not be able to remain still for long periods, such as children, or increase motion robustness for imaging participants with tremors. We are also interested in applying similar acceleration techniques to hyperpolarised xenon imaging of the lungs of healthy volunteers.Acknowledgements
Medical Research Council
Engineering and Physicsal Science Research Council
Oxford-Nottingham Biomedical Imaging Doctoral Training Centre.
References
1. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. 1. Magn Reson Med. 2007 Dec;58(6):1182-95.
2. Uecker M, Ong F, Tamir JI, et al. Berkeley advanced recon-struction toolbox. Proc Intl Soc Mag Reson Med Toronto.2015;23:2486.
3. Arthofer C, Safavi S, Cooper A, Harkin J, Alenazi S, Gowland P, Boltan C, Hall I. Impact assessment of posture and breath-hold state on diaphragm shape. Magn Reson Med conference paper. 2019
Figures