2358
Lung Function Assessment by Self-Gated 2D UTE – Comparison of Smoker and Non-Smoker1Department of Internal Medicine II, Ulm University Medical Center, Ulm, Germany, 2Core-Facility Small Animal Imaging, Ulm University, Ulm, Germany
Synopsis
In this study, a two-dimensional ultra-short TE protocol was used to acquire free-breathing data of the lung. The signal intensity in expiration and inspiration were used to derive the fractional ventilation, proton fraction and perfusion of the lung parenchyma.
Purpose
To investigate the feasibility of deriving lung functional parameters from self-gated ultra-short echo-time (UTE) MRI in smokers and non-smokers.Introduction
Ultra-short echo-time imaging has been applied for imaging of lung parenchyma, which is challenging for several reasons, including low signal due to the low proton density and the short T2* due to the frequent air-tissue interfaces in the lung parenchyma. [1] The UTE techniques are applicable for imaging of tissue with short T2* and have been successfully applied to lung imaging. [1–5] In this contribution, a self-gated UTE sequence has been applied for deriving the fractional ventilation, perfusion and proton density of the lung parenchyma in comparison of smokers and non-smokers.Methods
Eleven healthy volunteers (six non-smoker, five smoker) were examined on a 3T MR whole-body system (Achieva, Philips Healthcare, Best, The Netherlands). A two-dimensional ultra-short echo-time (2D UTE) protocol with the following parameters, TE=0.38ms, TR=2.3ms/2.6ms, flip angle=3.5°, slice thickness=20mm/10mm, tiny golden angle φ7 =23.62814° was applied to acquire three coronal slices (anterior, middle, posterior) during free breathing. The benefit of the tiny golden angle is a uniform coverage of the k-space for any number of projections thus supporting self-gating. [6] With the sliding window technique, low-resolution images were reconstructed from the free-breathing images. The respiratory gating signal was derived from the lung-liver interface as described in [3, 7]. The top 20% (expiration) and bottom 20% (inspiration) of the gating signal were used for the reconstruction of the different respiratory phases. With the signal intensity (SI) in the lung parenchyma ($$$SI_{lung}$$$) and blood vessels ($$$SI_{blood}$$$), the perfusion [8, 9] ($$$f = \frac{SI_{lung}}{SI_{blood}}\cdot \frac{1}{2\cdot T{exp}}$$$) was calculated, with $$$T_{exp}$$$ being the total experimental time divided by the number of heartbeats during that time. Fractional ventilation $$$FV$$$ was calculated as $$$FV= \frac{SI_{EX}-SI_{IN}}{SI_{EX}}$$$ [10] and the proton density $$$f_P$$$ according to $$$f_P=\frac{SI_{lung}}{SI_{muscle}}\cdot\exp(\frac{TE}{T2^*})$$$ (T2*=0.74ms) [4, 11].Results
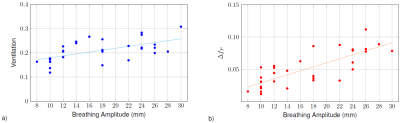
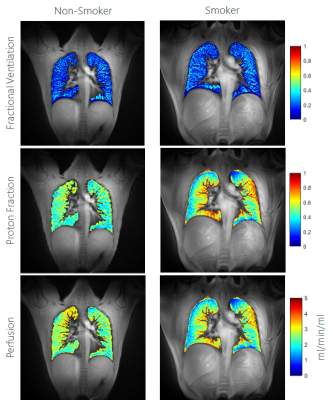
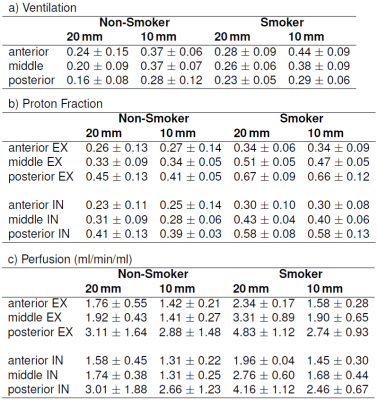
In all volunteers the proposed image-based self-gating provides good image quality, allowing the reconstruction of the lung parenchyma in expiration (EX) and inspiration (IN). Calculation of $$$FV$$$, $$$f$$$, and $$$f_P$$$ was possible in all cases. One case has to be excluded from the analysis due to low SNR in the inspiration image. As expected, a strong impact of the respiratory amplitude on the $$$FV$$$ and $$$f_P$$$ was observed (Figure 1) indicating the need for standardized breathing protocols. In the smoker as well as in the non-smoker group, deep and shallow breathing were observed. Analyses of the perfusion values showed a difference between EX and IN with significantly higher values during expiration and increasing perfusion from anterior to posterior (Table 1). No significant differences were observed for perfusion and $$$FV$$$ between the two groups. $$$f_P$$$ in expiration showed significantly higher values in the smoker group (Figure 2). Differences between the two different investigated slice thicknesses were observed. The ventilation resulted higher in the thinner slice, whereas perfusion values show opposite behaviour. The proton fraction resulted comparable between the two slice thicknesses.Discussion
This study proved the applicability of self-gated UTE for the quantification of functional lung parameters. The resulting values for the proton fraction, perfusion and fractional ventilation were comparable with the values in literature. [4, 9, 12, 13] The observed increase in the perfusion values from anterior to posterior was already described in Pracht et al. [12]. However, the observed reduction in perfusion with decreasing slice thickness is in contrast to Fischer et al. [9], but can most likely be explained by less vascular contributions in the thinner slices and the lower SNR. The rather weak R² for the ventilation can be explained by the fact that the ventilation values drop from anterior to posterior. No significant differences between smokers and non-smokers were noticeable for perfusion and fractional ventilation, which might be due to the large standard deviation caused by the different depth of breathing. The significant differences in the proton fraction during expiration (same respiratory position for all volunteers) between both groups indicate the performance of self-gated UTE as a sensitive measure for changes in the lung.Acknowledgements
The authors thank the Ulm University Center for Translational Imaging MoMAN for its support.References
1. TIBILETTI, Marta, KJØRSTAD, Asmund, BIANCHI, Andrea, SCHAD, Lothar R., STILLER, Detlef and RASCHE, Volker. Multistage self-gated lung imaging in small rodents. Magnetic resonance in medicine. 2016. Vol. 75, no. 6, p. 2448–2454.
2. BALASCH, Anke, METZE, Patrick, STUMPF, Kilian, SPEIDEL, Tobias and RASCHE, Volker. Self-Gated 2D UTE Lung Ventilation Imaging. In : Proc. Intl. Soc. Mag. Reson. Med. 27. 2019. 4086
3. TIBILETTI, Marta, PAUL, Jan, BIANCHI, Andrea, WUNDRAK, Stefan, ROTTBAUER, Wolfgang, STILLER, Detlef and RASCHE, Volker. Multistage three-dimensional UTE lung imaging by image-based self-gating. Magnetic resonance in medicine. 2016. Vol. 75, no. 3, p. 1324–1332.
4. HATABU, Hiroto, ALSOP, David C., LISTERUD, John, BONNET, Mathieu and GEFTER, Warren B. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. European journal of radiology. 1999. Vol. 29, no. 3, p. 245–252.
5. LEDERLIN, Mathieu and CRÉMILLIEUX, Yannick. Three-dimensional assessment of lung tissue density using a clinical ultrashort echo time at 3 tesla: a feasibility study in healthy subjects. Journal of Magnetic Resonance Imaging. 2014. Vol. 40, no. 4, p. 839–847.
6. WUNDRAK, Stefan, PAUL, Jan, ULRICI, Johannes, HELL, Erich and RASCHE, Volker. A small surrogate for the golden angle in time-resolved radial MRI based on generalized fibonacci sequences. IEEE transactions on medical imaging. 2014. Vol. 34, no. 6, p. 1262–1269.
7. PAUL, Jan, DIVKOVIC, Evica, WUNDRAK, Stefan, BERNHARDT, Peter, ROTTBAUER, Wolfgang, NEUMANN, Heiko and RASCHE, Volker. High-resolution respiratory self-gated golden angle cardiac MRI: comparison of self-gating methods in combination with k-t SPARSE SENSE. Magnetic resonance in medicine. 2015. Vol. 73, no. 1, p. 292–298.
8. KJØRSTAD, Asmund, CORTEVILLE, Dominique MR, FISCHER, Andre, HENZLER, Thomas, SCHMID-BINDERT, Gerald, ZÖLLNER, Frank G. and SCHAD, Lothar R. Quantitative lung perfusion evaluation using Fourier decomposition perfusion MRI. Magnetic resonance in medicine. 2014. Vol. 72, no. 2, p. 558–562.
9. FISCHER, André, PRACHT, Eberhard D., ARNOLD, Johannes FT, KOTAS, Markus, FLENTJE, Michael and JAKOB, Peter M. Assessment of pulmonary perfusion in a single shot using SEEPAGE. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008. Vol. 27, no. 1, p. 63–70.
10. KJØRSTAD, Asmund, CORTEVILLE, Dominique MR, HENZLER, Thomas, SCHMID-BINDERT, Gerald, HODNELAND, Erlend, ZÖLLNER, Frank G. and SCHAD, Lothar R. Quantitative lung ventilation using Fourier decomposition MRI; comparison and initial study. Magnetic Resonance Materials in Physics, Biology and Medicine. 2014. Vol. 27, no. 6, p. 467–476.
11. YU, Jiangsheng, XUE, Yiqun and SONG, Hee Kwon. Comparison of lung T2* during free-breathing at 1.5 T and 3.0 T with ultrashort echo time imaging. Magnetic resonance in medicine. 2011. Vol. 66, no. 1, p. 248–254.
12. PRACHT, Eberhard D., FISCHER, André, ARNOLD, Johannes FT, KOTAS, Markus, FLENTJE, Michael and JAKOB, Peter M. Single-shot quantitative perfusion imaging of the human lung. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2006. Vol. 56, no. 6, p. 1347–1351.
13. VOSKREBENZEV, A., GUTBERLET, M., WACKER, F. K. and VOGEL-CLAUSSEN, J. 3D lung ventilation 1H imaging using a respiratory self-navigated stack-of-stars sequence in comparison to 2D fourier decomposition. In : Proc. Intl. Soc. Mag. Reson. Med. 2016. p. 2913.
Figures