2345
Measuring Ventilation and Gas Exchange Rates with Hyperpolarized 129Xe During Tidal Breathing in Human Subjects
Hooman Hamedani1,2, Stephen J Kadlecek1, Faraz Amzajerdian 1,2, Ryan Baron1, Kai Ruppert1, Ian Duncan1, Yi Xin1,2, Mehrdad Pourfathi1, Sarmad Siddiqui1, Luis Loza1, Tahmina Achekzai1, Maurizio Cereda1,3, Rahim R. Rizi1, and fmig upenn4
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 4University of Pennsylvania, Philadelphia, PA, United States
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 4University of Pennsylvania, Philadelphia, PA, United States
Synopsis
A super multibreath maneuver to measure ventilation and gas exchange during tidal breathing in human subjects.
Purpose:
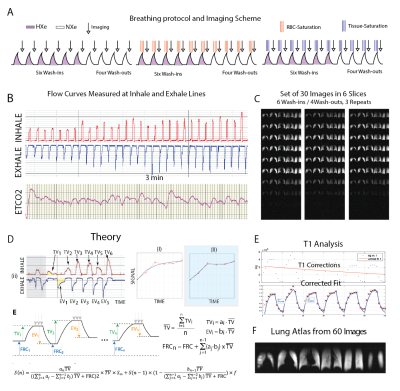
We previously showed the advantages of multi- over single-breath imaging of lung ventilation using hyperpolarized (HP) gas MRI1. Multi-breath imaging produces a comprehensive measure of ventilation (fractional ventilation-FV), which is closely related to the known physiological measure of ventilation (specific ventilation-SV), more closely mimics normal physiological breathing, allows the HP gas to reach slow-filling regions, and homogenizes the magnetization for imaging other physiological lung markers. Here, we further extend breathing sequence to allow acquisition of additional co-registered functional maps for straightforward multiparametric analysis. We also introduce a methodology to analytically correct for the unavoidable variability in the tidal volumes during such a sequence, which is a necessary step towards ability to image subjects during normal tidal breathing. In this example, gas dissolution was quantified on a regional basis by observing the loss of gas phase signal after selective saturation of either the tissue/plasma or the red blood cell (RBC) components (Xenon polarization Transfer Contrast (XTC) MRI2), yielding exchange rate maps. We also incorporated wash-out maneuvers in our breathing scheme as our simulations showed increased robustness to noise in estimation of FV/SV as well as these additional measurements.Methods and Results:
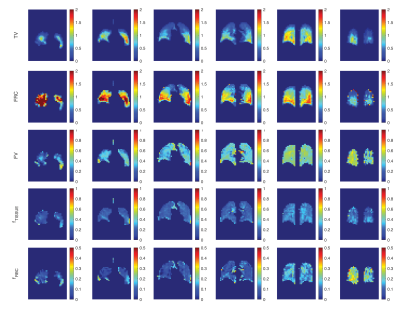
Despite a satisfactory precision of ±100 mL in trained healthy subjects1 for a 7 breath maneuver using our previous delivery system—which combines pneumotach-based monitoring of imaging gas and exhale gas flow with computer-controlled occlusion of the inhale sources at the target tidal volume—some variability in tidal breathing and residual volume persisted. Here, we eliminate the enforced occlusion and instead rely on repeated maneuvers and an analytical method to correct for this apparently unavoidable variability.In the new scheme, subjects inhale/exhale freely while HP gas is injected into the breathing line, and the number of wash-in/wash-out breaths is increased from 7 to 30-70 while keeping the total 129Xe volume the same. Images are acquired at end-exhale during a short pause (~2s) similar to that characteristic of tidal breathing. Figure 1A shows a schematic of the new breathing/imaging protocol. Subjects inhale/exhale with verbal guidance while gas flow rates and concentrations are being recorded (refer to Figure 1B). Coincident with each inhale, a constant amount of HP-Xe or non-polarized natural NXe or SF6 (~60mL) is injected into the line. We repeated sets of 6 wash-ins (HXe) and 4 wash-outs (NXe) until the supply of HXe was exhausted (Figure 1C). In the example shown here, a total of ~1.2L of HXe was used over 30 breaths. Notably, the scheme maintains a low Xe concentration of ~10-12%, reducing or eliminating the anesthetic/euphoric effects of the gas.To measure the additional markers relating to gas dissolution, as Figure 1A illustrates, a subset of the wash-in/wash-out maneuvers also included a series of 50 saturation pulses centered on the tissue/plasma or RBC resonances; the first set of 10 breaths contained no saturation pulses, while the second and third included saturation of RBC and tissue/plasma components immediately before imaging. As depicted in Figure 1D, measurements of inhale and exhale flows were used to fit the complete breath sequence in each voxel to a function that includes all dynamical effects at once. TV is the average measured breath volume over the entire sequence, a(j)TV / b(j)TV represent inhale/exhale volumes of the jth breath. All images will be sorted based on their signal level (in descending order), and in each step one image is registered to the one with higher signal and then will be added to that image to generate the base for the next registration. All these averaged pre-registered images produce a lung atlas. Subsequent computations will be on maps registered to this atlas. Figure 1E shows another set that was repeated 60 times in another representative subject by repeating the same sequence of Figure 1A. An exponential fit to the sums of signal can be used to correct for the longer effects of gas T1 in bag. Figure 2 shows the resulting maps of TV, FRC, FV, RBC and Tissue Loss.Conclusion:
We have developed an imaging scheme to measure regional ventilation (FV) and/or (TV and FRC) and XTC HXe gas exchange (RBC and Tissue) during normal breathing by diluting HXe to less than 10% and increasing the number of wash-in/wash-outs, and have successfully performed this technique in two healthy subjects.Acknowledgements
No acknowledgement found.References
1- Hamedani H. et al., Radiology 2016
2- Muradyan I. et al. Mag. Reson. Imag., 2013