2312
Applicability of automated cardiac phase sorting for phase resolved functional lung (PREFUL) MRI
Lea Behrendt1,2, Andreas Voskrebenzev1,2, Filip Klimeš1,2, Marcel Gutberlet1,2, Hinrich Winther1, Till Kaireit1,2, Tawfik Moher Alsady1,2, Gesa Pöhler1,2, Frank Wacker1,2, and Jens Vogel-Claussen1,2
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany
Synopsis
Phase-resolved functional lung (PREFUL) MRI is a promising contrast agent-free 1H method to derive information about perfusion dynamics in free breathing. The analysis includes the segmentation of a main vessel to determine the perfusion phase for reconstruction of a full cardiac cycle. Since manual segmentation can be challenging and time-consuming, an algorithm for automated phase sorting is desirable. In this study such an algorithm is proposed, tested in 34 patients with CF and CTEPH and compared to manual phase sorting. The algorithm was able to perform segmentation in all cases and showed improved phase sorting compared to manual segmentation.
Introduction
Phase resolved functional lung (PREFUL) MRI1 is a contrast agent free 1H technique, allowing for simultaneous assessment of lung ventilation2 and perfusion3 dynamics. It is based on the voxel-wise time series analysis of dynamic lung MRI in free breathing4. To derive dynamic information about pulmonary ventilation and perfusion, lung images are co-registered, low-pass or high-pass filtered to extract the ventilation or perfusion components and retrospectively sorted to reconstruct one respiratory and cardiac cycle. During PREFUL postprocessing, the cardiac phase is determined using the mean signal time series in a manual segmented region of interest (ROI) inside a main pulmonary vessel1. However, manual segmentation is time-consuming, requires user interaction and is challenging especially in posterior and anterior slices of the lung. Therefore, in this study, an algorithm for automated cardiac phase sorting is proposed. The applicability of the developed algorithm was tested in patients with chronic lung disease in slices covering the whole lung.Method
14 patients with cystic fibrosis (CF) and 20 patients with chronic thromboembolic pulmonary hypertension (CTEPH) were included in this study. Imaging was performed on a 1.5T scanner (Avanto or Aera, Siemens Healthcare, Erlangen, Germany) using a spoiled gradient echo sequence with the following settings: Field of view = 225 x 225mm2 - 500 x 500mm2, matrix size 128 x 96 - 128 x 128 (all interpolated to 256 x 256), slice thickness 15mm, slice spacing 15mm - 30mm, TE = 0.67ms - 0.91ms, TR = 3ms, flip angle 5° - 8°, temporal resolution 192ms - 289ms. Between 4 and 13 coronal slices covering the whole lung were obtained for each patient. All images were registered towards one fixed image in intermediate lung position5. Afterwards, an automated algorithm for cardiac phase sorting inside PREFUL1 was implemented (Figure 1): Therefore, an ROI (Rsort), consisting of at least one voxel cluster, located inside a pulmonary vessel or the heart shall be segmented automatically. First, after automatic segmentation of the lung parenchyma using a semantic convolutional neural network6, a search ROI (As) consisting of both lungs and the mediastinum was created. Perfused vessels and the heart, provide a high signal intensity and a large amplitude in the cardiac frequency spectrum. Therefore, voxel clusters inside vessels and the heart were identified from a temporal maximum intensity projection map MMIP and a standard deviation map Mstd of the high-pass filtered (cut-off frequency: 0.75Hz) dynamic image series using the 98th percentile as the lower threshold. To avoid cardiac phase shifts between the voxel clusters and to improve the sine fit during reconstruction of the cardiac cycle, Rsort was iteratively adjusted by comparing the goodness of fit parameter R2 of the acquisitions fitted to the reconstructed sine wave before and after removing a cluster (Figure 2). If the sine fit improves, the cluster will be removed from Rsort. As a lower limit, at least one cluster had to remain in Rsort. Finally, images were sorted according to their perfusion phase and interpolated to 15 phases at an equidistant time grid covering one cardiac cycle. R2 values of the sine fit were calculated for all slices and compared to those obtained with manually segmented ROIs inside a main vessel using a paired two-sided Wilcoxon test.Results
For every slice, a sorting ROI inside a vessel or the heart was found by the algorithm. In Figure 3, segmented ROIs, corresponding sine fits and perfusion-weighted maps obtained using the automatic algorithm and manual segmentation are shown for selected slices of a CTEPH patient. Perfusion-weighted maps obtained by the algorithm are comparable or, especially for anterior slices without large vessels, improved to those obtained by manual segmentation. R2 values for all slices are shown in Table 1. Including all patients, median R2 increased from 0.81 (0.71-0.88) using manual segmentation to 0.85 (0.79-0.90) using the automatic algorithm (P<0.001). For the CF patients, R2 increased from 0.86 (0.75-0.91) to 0.88 (0.83-0.91) (P<0.001) and for the CTEPH patients, R2 increased from 0.79 (0.70-0.85) to 0.84 (0.77-0.89) (P<0.001).Discussion
Applicability of automated cardiac phase sorting was successfully tested in CF and CTEPH patients with similar or improved results of further PREFUL analysis compared to manual segmentation. Even at posterior and anterior slices containing only small vessels, where manual segmentation is difficult, the algorithm could still segment a proper ROI inside a vessel or the heart. Limitations of the algorithm occurred solely for peripheral posterior slices, where additional spinal ROI clusters were found. However, for those slices, manual segmentation was almost unfeasible. Further, functional maps generated for those slices, are often not diagnostically conclusive due to through-plane motion and partial volume effects. Considering the significantly increased coefficient of determination in comparison to the manual evaluation, the algorithm may help to improve pulmonary perfusion assessment using PREFUL.Conclusion
The algorithm presented in this study allows for automated cardiac phase sorting of coronal slices covering the whole lung during PREFUL postprocessing. This is an important step towards a completely automated PREFUL analysis.Acknowledgements
No acknowledgement found.References
- Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med 2018;79:2306-2314.
- Klimeš F, Voskrebenzev A, Gutberlet M, et al. Free‐breathing quantification of regional ventilation derived by phase‐resolved functional lung (PREFUL) MRI. NMR in Biomedicine 2019;32:e4088.
- Kaireit TF, Voskrebenzev A, Gutberlet M, et al. Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients. J Magn Reson Imaging 2019;49:1122-1132.
- Bauman G, Puderbach M, Deimling M, et a. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn Reson Med 2009;62:656-664
- Voskrebenzev A, Gutberlet M, Kaireit TF, et al. Low-pass imaging of dynamic acquisitions (LIDA) with a group-oriented registration (GOREG) for proton MR imaging of lung ventilation. Magn Reson Med 2017;78:1496-1505.
- Winther H, Gutberlet M, Hundt C, et al. Deep semantic lung segmentation for tracking potential pulmonary perfusion biomarkers in chronic obstructive pulmonary disease (COPD): The multi‐ethnic study of atherosclerosis COPD study. J Magn Reson Imaging doi: 10.1002/jmri.26853
Figures
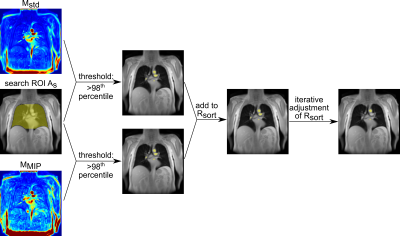
Figure
1: Region of interest (ROI) detection of the automated algorithm for cardiac phase sorting of PREFUL MRI. Voxel clusters
inside the search ROI (As) and above defined thresholds of a
perfusion weighted map (Mstd) and a temporal maximum intensity
projection map (MMIP) are considered for the phase sorting ROI (Rsort).
Rsort is iteratively adjusted to improve the reconstruction of the
cardiac cycle.

Figure 2:
Reconstruction of full cardiac cycle of the automated algorithm for cardiac phase sorting of PREFUL MRI. First, a signal time series is obtained from
the segmented ROI (red). Then, a piecewise sine fit (red/ blue lines) is
applied to the signal and the phase is determined to reconstruct a sine wave
and thus a full cardiac cycle.
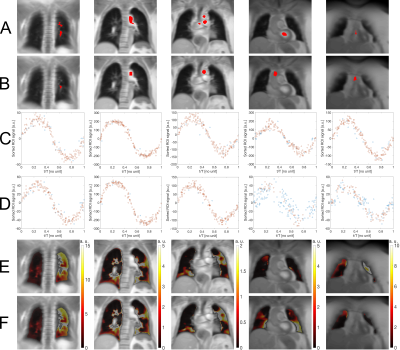
Figure
3: Segmented ROIs (red) for phase sorting for posterior to anterior slices of a
CTEPH patients using (A): the automatic algorithm. (B): manual segmentation.
And corresponding sine fits using (C): the algorithm. (D): manual segmentation.
R2 >0.9 of fitted acquisitions in the corresponding sine fits are
marked with a red circle and R2<0.9 are marked with a blue
circle. Perfusion-weighted maps (E): calculated after applying the algorithm.
(F): obtained after using manual segmentation.

Table
1: Comparison of goodness of fit parameter R2 for manual and
automatic cardiac phase sorting.
All
patients included and slices sorted by distance to a tracheal slice located at
the aortic arch. Data provided as median with 25th and 75th
percentiles. Improved R2 values printed in bold.