2311
Quantification of Hyperpolarized 129-Xe signal fluctuations in a rodent chest restriction model1UPenn, Philadelphia, PA, United States, 2Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 4Surgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We demonstrated a new imaging technique capable of quantifying Xe gas uptake into airways and parenchyma over the course of a breathing cycle. Most current imaging techniques acquire entire images at a single breathing point. These methods may not replicate organ function during tidal breathing, and could potentially miss diagnostically important dynamic information depending on the time of acquisition. This sequence traverses the k-space over a series of 576 breaths to produce 12 images at different time points. Three healthy rats were imaged, and Xe signal was found to fluctuate at different times and amplitudes depending on respective lung compartment.
Introduction
The development of scoliosis at an early age often leads to severely compromised lung function, as the rotation and displacement of the spinal structure can cause a decrease in lung volume, displace organs and interfere with muscle and rib mechanics 1. Current clinical assessments of scoliosis severity rely on detecting changes in lung volume but are not able to quantify the associated change in organ function. In this study, we applied a newly developed imaging technique to a rodent model of scoliosis-induced restrictive lung disease in order to understand how gas dynamics over the course of a breathing cycle may be affected by spinal curvature, causing changes in ventilation and perfusion.Methods
Chest-restriction surgery was performed on 15 Sprague-Dawley rats (approx. 50 g) by tethering ribs 3-9 on the right side of the chest without disturbing lung parenchyma. 3D Micro-CT imaging was used to measure Cobb angles to assess the degree of scoliosis post-surgery.After growing to 200-300g (approximately 5 weeks post-surgery), three healthy and three scoliotic rats were imaged with 129-Xe MRI. Cobb angle measurements for the scoliotic animals were typically 30o. All rats were ventilated with 30%/70% oxygen/nitrogen except during imaging, when the N2 was replaced by 129-Xe. Tidal volume was constant and set equal to the Functional Residual Capacity as determined by pulmonary function tests performed prior to imaging (DSI/Buxco PFT).
All MRI studies were performed in a whole body 1.5-T scanner (Avanto; Siemens). A custom Xe-129 transmit/receive birdcage coil was used (Stark Contrast, Erlangen, Germany). Enriched xenon gas (87% Xe-129) was polarized in a prototype commercial system (XeBox-E10, Xemed LLC, Durham, NH). All studies were approved by the Institutional Animal Care and Use Committee.
A 24x24 2D-CSI was acquired over 576 breaths, repeatedly acquiring a single k-space point 12 times over the course of each breath cycle to produce twelve cine 129-Xe images corresponding to time points equally spaced by 106ms; this enabled dynamic visualization of each of the three primary 129Xe components: Xe in the gas, tissue/plasma and hemoglobin-bound compartments. To quantify these differences, the images at different points of the breathing cycle were registered to each other using affine transformations and mapped back to the end-exhale image. Signal values were quantified for each compartment, spatial location and time. Signal dynamics at a specific location were then summarized by the mean value (‘S’), the extent of variation (‘ΔS’), and the phase of the cyclical variation (‘θ’) during the breathing cycle. θ was calculated by performing a Fourier transform of an individual voxel’s signal values over time and evaluating the phase of the lowest non-zero frequency component, corresponding to the breathing rate.
Results and Discussion
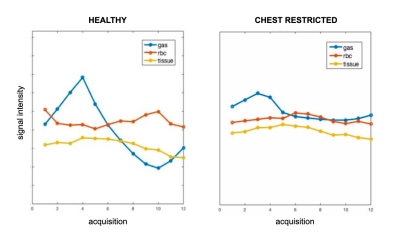
Figure 1 shows the change in total Xe signal in gas, tissue and blood over the course of 12 points in the breathing cycle in both a healthy rat (A) and in a representative restricted rat (B). In the healthy rat, the gas signal initially increases, peaking at end-inhale. The signal of Xe dissolved in tissue/plasma continues to increase for about 300 ms afterwards, while the signal of Xe bound to blood cells reaches maximum shortly before end exhale. The gas-phase Xe in the restricted rat also reaches maximum at end-inhale, as expected. However, the delay between the signal peaks of Xe dissolved in tissue and bound to hemoglobin is almost eliminated. Across the entire cohort, the mean delay between peak gas and peak hemoglobin-bound signals was 459 +/- 162 ms in healthy rats, but was significantly reduced, to 177 +/- 122 ms, in the restricted cohort.In Figure 2, the ΔS and θ maps in a healthy and a restricted rat are compared. The healthy rat exhibits the characteristic delay between the peak signal of Xe in tissue and that bound to RBC observed in the global plots. This is evident in the contrast between the θ maps of tissue and RBC signal: the tissue map displays a mean phase of 4.2 radians, while the RBC has a mean phase of about 5.9 radians. In the chest restriction model, the mean phase of the Xe in tissue and RBC are much closer, at 3.6 and 4.0 radians, respectively. These results suggest that cyclic variability in pulmonary blood volume is drastically reduced in the restriction model. This early plateau in RBC signal suggests that the spinal curvature affects the blood flow in restricted rats, limiting the influx of blood at end-exhale that is typical of the healthy organ.
Conclusion
We demonstrated the utility of spectrally selective 129Xe MR imaging to studying differences in gas dissolution dynamics in the lung. Our results suggest that this technique may be an important tool for probing altered pulmonary dynamics in the case of disease.Acknowledgements
No acknowledgement found.References
(1) Tsiligiannis, T., & Grivas, T. (2012). Pulmonary function in children with idiopathic scoliosis. Scoliosis, 7(1), 7.Figures
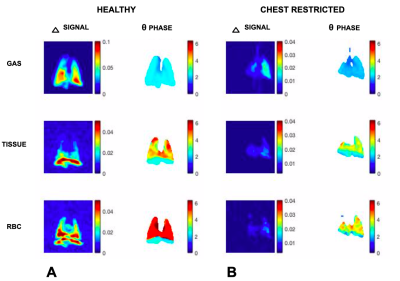
Δ and θ maps in a representative healthy rat compared to a representative chest restricted rat, for Xe in gas phase, dissolved in tissue, and bound to red blood cells (top to bottom). The maps demonstrate a much stronger variation in tissue versus RBC in the healthy model as opposed to the chest-restricted rat.