2302
Reproducibility of functional lung MRI using non-contrast-enhanced 3D-UTE MRI in breath-hold technique1Department of Diagnostic and Interventional Radiology, University Hospital, Würzburg, Germany, 2Application Development, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Functional and morphologic assessment of lung disease is to date performed separately using pulmonary function testing and mostly radiation-based imaging. UTE MRI is a promising tool for radiation-free pulmonary imaging. Pre-existing invasive functional MRI techniques have been transferred to UTE MRI. For non-invasive combined functional and morphologic imaging, radiation-free 3D-UTE MRI acquired in breath-hold and using stack-of-spirals trajectories enables ventilation measurements with high reproducibility for both tidal and deep fractional ventilation.
Introduction
To date, functional and morphologic assessment of lung diseases is performed separately using pulmonary function testing and chest radiography or computed tomography (CT). In order to reduce cumulative radiation dose especially in chronic pulmonary diseases, MRI using ultrashort echo times has been demonstrated to provide an image quality similar to CT1-3. Compared to former sequence techniques used for pulmonary MRI, UTE sequences allow for significantly improved signal yield. Pre-existing functional MR imaging techniques, for example oxygen-enhanced imaging and contrast-enhanced perfusion imaging, have been transferred to pulmonary UTE MRI in scientific setups4,5 in order to combine functional and morphologic pulmonary imaging. However, non-invasive combined functional and morphologic imaging is the declared goal in the development of radiation-free functional lung MRI. For this purpose, the aim of this work was to evaluate the intraindividual reproducibility of functional lung analysis using non-contrast-enhanced 3D-UTE MRI.Methods
Nine healthy volunteers without acute or known chronic pulmonary disease were included into this prospective single-center study and underwent contrast-free 3D-UTE MRI in breath-hold technique. Imaging was repeated within five to eight days. MR image sets were acquired on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a prototypical stack-of-spirals 3D-UTE sequence6 with a dual-density trajectory7, iPAT factor 2, and further sequence parameters as follows: TR = 2.35 msec; TE = 0.05 msec; flip angle = 5°; FOV = 600 mm x 600 mm; in-plane resolution = 2.3 mm x 2.3 mm; slice thickness = 2.3 mm; nonselective hard pulse duration = 60 µs; spiral interleaves = 264; readouts per spiral = 265; number of partitions = 102 ± 14 (depending on the thoracic diameter). Five different breathing states (deep inspiration, normal inspiration, breathing baseline, normal expiration and deep expiration) were acquired within a single breath-hold of 12.7 – 17.6s each, resulting in an overall acquisition time of less than 3 minutes. Images were reconstructed using SPIRiT reconstruction8. Breathing states were registered to the breathing baseline dataset using a deformable B-spline registration algorithm9. The signal intensities (SI) of all acquired breathing states were plotted voxel-wise, and a linear fit was performed to model the decreasing signal intensity from deep expiration to deep inspiration in order to minimize the impact of image noise on the data. Finally, fractional ventilation (FV) was calculated according to Zapke et al10. Further, FV was calculated as change of lung volumes (Vol) over the breathing cycle as well10.Results
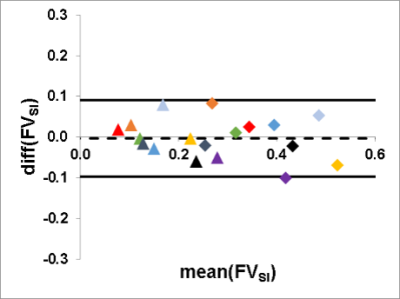
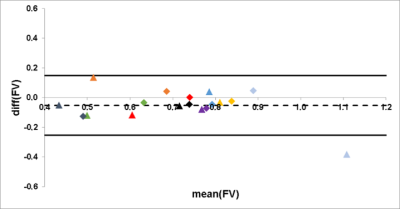
FVSI of tidal ventilation with mean values of 0.16 ± 0.06 ml air/ml lung parenchyma for the first examination and 0.17 ± 0.08 ml air/ml lung parenchyma for the second one showed high correlation with a Spearman´s correlation coefficient of r = 0.87. Assessment of FVSI for deep ventilation yielded a mean of 0.38 ± 0.08 ml air/ml lung parenchyma for the first and 0.38 ± 0.10 ml air/ml lung parenchyma for the second scan, with good correlation (r = 0.85). Bland-Altman Analysis showed a mean difference of 0.00 with an interval of confidence (CI) ranging from -0.09 to 0.09 (Figure 1). The difference of the ventilation parameters FVSInorm and FVSIdeep was significant (p < 0.01). In order to decouple FVSI from the breathing depth FVSI was divided by FVVol. This led to an adaption of FV for tidal (first scan, 0.66 ± 0.16 ml air/ml lung parenchyma; second scan, 0.77 ± 0.24 ml air/ml lung parenchyma) and deep ventilation (first scan, 0.72 ± 0.13 ml air/ml lung parenchyma; second scan, 0.75 ± 0.10 ml air/ml lung parenchyma; p = 0.25), with high correlation for tidal (r = 0.85) and deep ventilation (r = 0.97). Mean difference of Bland-Altman Analysis was 0.05 with a CI from -0.25 to 0.15 (Figure 2).Discussion
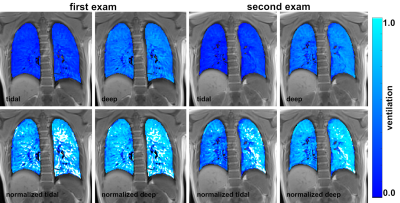
The assessment of both, FVSI of tidal and deep ventilation showed high reproducibility of calculated fractional ventilation using 3D-UTE MRI. Mean FVSI values acquired in deep ventilation were approximately twice as high as in tidal ventilation, confirming the strong dependence of FVSI from breathing depth. Normalizing FVSI decoupled the value for fractional ventilation from breathing depth and still showed high reproducibility (Figure 3). Mean values of normalized FV <1.0 can be attributed to image artifacts, large pulmonary vessels or inaccuracies during segmentation of the lungs and resulting errors in calculation of lung volumes.Conclusion
Non-contrast-enhanced 3D-UTE MRI allows for morphologic imaging as shown before and highly reproducible determination of tidal and deep fractional ventilation in breath-hold technique. It is supposed to be a promising tool for monitoring of respiratory function, for example in patients with chronic lung disease or during lung toxic therapies.Acknowledgements
The project underlying this report was funded by the Deutsche Forschungsgemeinschaft (DFG).
The Department of Radiology receives a research grant from Siemens Healthcare GmbH. The grant is not specifically directed towards any of the authors.
References
1. Wielputz MO, Triphan SMF, Ohno Y, Jobst BJ, Kauczor HU. Outracing Lung Signal Decay - Potential of Ultrashort Echo Time MRI. Rofo. 2019;191(5):415-23.
2. Ohno Y, Koyama H, Yoshikawa T, Kishida Y, Seki S, Takenaka D, et al. Standard-, Reduced-, and No-Dose Thin-Section Radiologic Examinations: Comparison of Capability for Nodule Detection and Nodule Type Assessment in Patients Suspected of Having Pulmonary Nodules. Radiology. 2017;284(2):562-73.
3. Wielputz MO, Lee HY, Koyama H, Yoshikawa T, Seki S, Kishida Y, et al. Morphologic Characterization of Pulmonary Nodules With Ultrashort TE MRI at 3T. AJR Am J Roentgenol. 2018;210(6):1216-25
4.Kruger SJ, Fain SB, Johnson KM, Cadman RV, Nagle SK. Oxygen-enhanced 3D radial ultrashort echo time magnetic resonance imaging in the healthy human lung. NMR Biomed. 2014;27(12):1535-41.
5. Bannas P, Bell LC, Johnson KM, Schiebler ML, Francois CJ, Motosugi U, et al. Pulmonary Embolism Detection with Three-dimensional Ultrashort Echo Time MR Imaging: Experimental Study in Canines. Radiology. 2016;278(2):413-21.
6. Mugler JP MC, Pfeuffer J, Stemmer A, Kiefer B. Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging. Proc Intl Soc Mag Reson Med 2017:4904.
7. Meyer CH ZL, Lustig M, Jilwan-Nicolas M, Wintermark M, Mugler JP, Epstein FH. Dual-Density and Parallel Spiral ASL for Motion Artifact Reduction. Intl Soc Mag Reson Med. 2011;64:3986.
8. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64(2):457-71.
9. Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196-205.
10. Zapke M, Topf HG, Zenker M, Kuth R, Deimling M, Kreisler P, et al. Magnetic resonance lung function--a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir Res. 2006;7:106.
Figures