2300
Imaging of lung parenchyma: comparison of UTE3D, ZTE3D vnav, and ZTE4D acquisitions1Radiology and Nuclear Medicine, Erasmus Medical Center, Rotterdam, Netherlands, 2Pediatrics, Erasmus Medical Center, Rotterdam, Netherlands, 3GE Healthcare, Hoevelaken, Netherlands
Synopsis
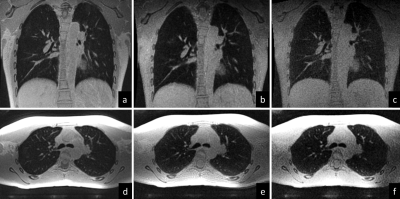
MRI has recently emerged as a potential clinical tool that can produce high resolution images of structural changes in the lung thanks to the use of ultrashort TE readouts without the ionizing radiation of CT scans [3-6]. Thanks to this developments, pediatric cystic fibrosis (CF) patients can undergo routine monitoring with CT like quality [1]. In this study we investigate three different MRI sequences (UTE, ZTE3D vnav and ZTE 4D). In general, all sequences perform reasonably well though ZTE3D vnav seems to provide the best performance for CF monitoring.
Introduction
Owing to its high spatial resolution and acquisition speed, CT is commonly used for depicting structural lung changes . Nonetheless, CT uses ionizing radiation, which might be harmful for developing cell, such those of children, who are at higher risk of developing cancer, related to radiation exposure. Several pediatric lung diseases require constant monitoring, which is nowadays mostly performed through chest x-ray or CT [1]. The most common inherited lung disorder in Caucasian is CF, which is characterized by progressive lung damage until end-stage lung disease. Constant monitoring with lung function and imaging is required to slow down lung damage and delay lung transplant [2]. MRI has recently emerged as a clinical tool to image the lungs [3] providing the opportunity to monitor CF pulmonary changes without radiation at high resolution. This has been possible with the introduction of ultrashort TE readouts, such as UTE and the silent variant ZTE that enable the depiction of small intrapulmonary structures and peripheral airways by counteracting the T2* effect of air in the lungs. In this study we investigated three available sequences, namely UTE3D, ZTE3D vnav and ZTE4D in normal volunteers and in pediatric CF patients.Materials and methods
Scanning and image comparison was performed on 12 healthy volunteers (18-38) and 5 CF patients (7-16) on a 1.5T MRI scanner (Signa Artist, GE Healthcare) using three ultrashort TE readout schemes with a target isotropic voxel resolution of 1.5x1.5x1.5 mm3. Axial volumes were prescribed. A 32 channel torso array coil was used for signal reception. UTE3D used a 3D cones trajectory with a readout bandwidth of 125KHz gated at end expiration with a respiratory pneumobelt. The silent ZTE3D vnav used a readout bandwidth of 62.5KHz and was navigated using a projection and gated to end expiration. A continuous silent acquisition, ZTE4D, was used in conjunction with the pneumobelt respiratory signal [8] in order to obtain one phase (end expiration) or all phases of the respiratory cycle if needed (16 max) . The ZTE readouts used a radial spokes trajectory. Volunteer and patients were instructed to breath as regularly as possible. On CF patients, a 6-8s breath-hold RF spoiled 3D gradient echo scan (SPGR3D) was acquired in maximum inspiration and expiration for comparison (voxel resolution of 3.0x3.0x3.0 mm3 with TE=0.6ms). The readout flip angle for all sequences was chosen to obtain a proton density image contrast with a CT lung-window like appearance, where muscle and fat have similar signal intensity and contrast. Patients underwent CT scan within 2 weeks from the MRI.Results
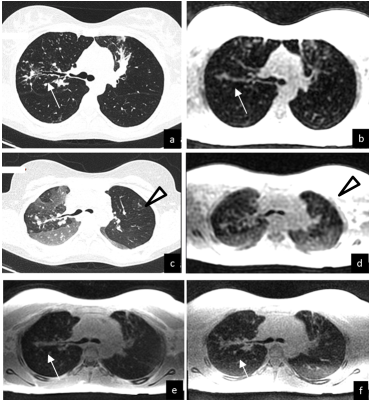
Due to their acquisition characteristics, differences between the sequences did arise. UTE3D had a longer repetition time than the ZTE counterparts leading to longer scan times. Nonetheless, the lower flip angle used for the ZTE scans required more projections and more averaging in order to provide the same image quality (SNR) therefore making scan times similar. The overall scanning time therefore was similar for all sequences. Figure 1 demonstrates a scan on a volunteer. . Despite similar resolution, the ZTE4D readouts provided constantly lower image quality, with lower SNR and more grainy appearance of the images. Background noise between UTE and ZTE was different, where ZTE was providing a more whitish appearance of the lung parenchyma. Figure 2 depicts a CF patient comparing breath-hold inspiratory and expiratory CT with breath-hold low resolution SPGR3D scans using an MR compatible spirometer with the expiratory gated ultrashort TE readouts. One obvious difference that arose between all sequences was when comparing them against the expiratory CT – the depiction of air trapping /regions of low image density. In general, all structures could be well seen on all ultrashort TE sequences. The ZTE variants were preferred because of the quietness and comfort for patients.Discussion
This is the first study comparing UTE and ZTE imaging both with prospective and retrospective gating to CT for lung imaging. Our study showed that both sequences perform equally well. Interestingly the retrospective gating version of ZTE (ZTE4D@GE) did not provide reliable image quality. This was unexpected, because we thought that a continuous acquisition during the respiratory cycle could have enabled a better reorder of the respiratory phases. This approach remains anyway promising, because it might enable both inspiratory and expiratory imaging in a single acquisition. Improvement of the silent ZTE4D might be useful to extend the use of lung MRI to neonates, who are sensitive to noise and has high respiratory rates (range 40-80 cycles/min versus 12-15 cycles/min adults). Breath-hold UTE/ZTE imaging might overcome the only limitation noticed in our study regarding UTE/ZTE readouts, which was the detection of areas of air trapping. New k-space acquisition scheme or the combination with new parallel imaging technique (Compress sensing) might allow to close the gap between MRI and CT, making UTE/ZTE readouts a robust alternative to CT [9].Conclusions
UTE and ZTE readouts provide similar image quality for lung MRI to assess structural changes. Conversely, detection of low density region is limited both in UTE and ZTE compared to CT, likely due to free-breathing condition. Short TE breath-hold SPGR scan still provide the best CNR to detect air trapping with MRI.Acknowledgements
No acknowledgement found.References
[1] Leuraud K, Richardson DB, Cardis E et al (2015) Ionising radiation and risk of death from leukaemia and lymphoma in radiationmonitored workers (INWORKS): an international cohort study. Lancet Haematol. doi:10.1016/S2352-3026(15)00094-0
[2] Jain M, Goss CH (2014) Update in cystic fibrosis 2013. Am J Respir Crit Care Med 189:1181–1186
[3] Wild JM, Marshall H, Bock M, Schad LR, Jakob PM, Puderbach M, et al. MRI of the lung (1/3): methods. Insights Imaging. 2012;3(4):345-53. https://doi.org/10.1007/s13244-012-0176-x
[4] Robson, Matthew & Gatehouse, Peter & Bydder, Mark & Bydder, Graeme. (2003). Magnetic Resonance: An Introduction to Ultrashort TE (UTE) Imaging. Journal of computer assisted tomography. 27. 825-46. 10.1097/00004728-200311000-00001.
[5] Reichert IL, Benjamin M, Gatehouse PD et al (2004) Magnetic resonance imaging of periosteum with ultrashort TE pulse sequences. J Magn Reson Imaging 19:99–107
[6] Weiger, M. , Brunner, D. O., Dietrich, B. E., Müller, C. F. and Pruessmann, K. P. (2013), ZTE imaging in humans. Magn. Reson. Med., 70: 328-332. doi:10.1002/mrm.24816
[7] Bae, Kyungsoo & Jeon, Kyung-Nyeo & Hwang, Moonjung & Lee, Joon & Ha, Ji & Ryu, Kyeong & Kim, Ho Cheol. (2018). Comparison of lung imaging using three-dimensional ultrashort echo time and zero echo time sequences: preliminary study. European Radiology. 10.1007/s00330-018-5889-x.
[8] Self-Calibrated Soft Gating for Respiratory Resolved 3D+Time Lung ZTE ISMRM 2018, Menini, Lai, McKinnon, & Wiesinger.
[9] MR Imaging of Lungs and Airways in Children:: Past and Present. Liszewski MC, Ciet P, Lee EY. Magn Reson Imaging Clin N Am. 2019 May;27(2):201-225
[10] Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Dournes G, Menut F, Macey J, Fayon M, Chateil JF, Salel M, Corneloup O, Montaudon M, Berger P, Laurent F. Eur Radiol. 2016 Nov;26(11):3811-3820. Epub 2016 Feb 2. MAGMA. 2015 Jun;28(3):207-15. doi: 10.1007/s10334-014-0459-y. Epub 2014 Sep 9.
[11] Free-breathing, zero-TE MR lung imaging. Gibiino F1, Sacolick L, Menini A, Landini L, Wiesinger F.
Figures