2298
Dynamic 3D Ventilation Maps with Phase-Resolved Ultrashort Echo Time (UTE) MRI
Fei Tan1, Xucheng Zhu1, and Peder E. Z. Larson1,2
1Graduate Program in Bioengineering, UC Berkeley - UCSF, San Francisco, CA, United States, 2Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
1Graduate Program in Bioengineering, UC Berkeley - UCSF, San Francisco, CA, United States, 2Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
In this abstract, we propose a novel respiratory phase-resolved reconstruction for free-breathing pulmonary 1H UTE MRI. We compute the tissue deformation-based ventilation maps and further use the parametric and non-parametric fitting methods to reduce the noise and registration errors.
Introduction
Recent functional analysis using 1H MRI demonstrates its ability to resolve ventilation information. For example, Fourier decomposition (1) extracts ventilation-induced lung density change from intensity and frequency information. Phase-resolved functional lung (PREFUL) MRI (2) utilize the same information but reconstructs a full respiratory cycle to get the ventilation volume and relative timing to the diaphragm. However, these methods primarily deal with 2D free-breathing images and need longer scan times if multiple slices are of interest. 3D ventilation analysis methods such as UTE-based self-gated non-contrast-enhanced functional lung (UTE-SENCEFUL) MRI (3) are also intensity change-based analysis. An alternative way to calculate volume change is to use the tissue deformation, as mentioned in (4). To date, these methods require breath-hold which may lead to subject discomfort. Building on the motion-resolved method (5) and the hysteresis nature of the lung, we propose a phase-resolved 3D UTE MRI. By using the tissue deformation information of each respiratory phase, we can generate dynamic ventilation maps. Parametric and non-parametric fitting methods are incorporated to reduce noise introduced by imperfect reconstruction and registration.Methods
5 healthy volunteers (age 26-30, 2 females 3 males) were included in this study. All images were acquired on a 3T MR750 clinical scanner (GE Healthcare, Waukesha, WI) with an 8-channel cardiac phased-array coil. An SNR optimized 3D radial UTE sequence (6) with golden-angle ordering (7) (Fig.1a) was adopted during free-breathing (FOV = 32 - 40cm, pixel size = 2.5mm isotropic).Firstly, we implement phase-resolved reconstruction to separate the respiratory cycle into the desired number of phases instead of grouping data based on respiratory signal intensity (5). We use the center of k-space for motion detection (7,8) and determine the phase within a respiratory cycle. The corresponding acquisition spokes are then assigned to different bins followed by non-Cartesian reconstruction (5). (Fig. 1b-c)
Each phase-resolved frame is registered to the end-expiration frame by non-rigid registration via Advanced Normalization Tools (ANTs) (9,10). Then, the Jacobian determinant (JD) is calculated using the motion field derived from the registration (Fig. 1d). The Jacobian determinant is defined as
$$JD = \frac{V_{phase_i}}{V_{expi}} = \left |\det \left [Id + \frac{\partial (D_x, D_y, D_z)}{\partial (x,y,z)}\right]\right|$$
where $$$V_{expi}, V_{phase_i}$$$ are the volume at end-expiratory and the ith respiratory phase. $$$D$$$ is the deformation field, $$$x,y,z$$$ are the spatial dimensions and $$$Id$$$ is the 3-by-3 identity matrix. Since the motion fields among respiratory phases should change smoothly, we apply a voxel-by-voxel 3rd order polynomial fitting on either the Jacobian determinant or the motion field across time (Fig. 1e).
Another underlying assumption is that the volume change across the spatial domain should also be continuous. The redundant information from the spatial domain can help retrieve the realistic volume change. We applied singular value decomposition in the spatial and temporal domain and recovered the data for ventilation analysis with only two principal components.
Results
Figure 2 shows the phase-resolved MRI of three healthy volunteers. The respiratory cycle is separated into 6 phases. Note that the diaphragm position is slightly different for the phases immediately before and after the end-expiration state. This indicates that the phase-resolved reconstruction can account for hysteresis during inhalation and exhalation. The sharp edge across lung and diaphragm symbols an effective k-space radial trajectory binning.Figure 3 illustrates the ventilation maps calculated by Jacobian determinant, Jacobian determinant fitting, motion field fitting, and singular value decomposition of different respiratory states. The right column plots the average ventilation curve of each lobe versus the respiratory phase. Jacobian determinant fitting and motion field fitting yield similar ventilation maps and curves. Both methods smooth the ventilation curves, but the accuracy of these parametric fittings may rely on the polynomial order selection and the ordering of the respiratory phases. The singular value decomposition provides ventilation maps and curves that resemble the original ones that are smoothed in both temporal and spatial dimensions.
Figure 4 demonstrates ventilation maps of the same volunteer across interleaved tidal breathing and diaphragmatic breathing. The volume change of diaphragmatic breathing is significantly larger than that of the tidal breathing, while the expanding regions are correlated. The images suggest that phase-resolved reconstruction and fitted Jacobian determinant ventilation map calculation can be applied to extreme breathing conditions.
Discussion & Conclusion
The phase-resolved UTE MRI has the potential of enabling various ventilation analysis metrics, especially timing-based methods. The fitted Jacobian method utilizes smoothness prior knowledge to correct the imperfections induced by reconstruction and registration. In the future, we aim at developing other ventilation quantification methods using the volume curve of each voxel in the phase-resolved image and compare to the existing fitted Jacobian determinant approach.Acknowledgements
This work is supported by NIH grant R01 HL136965.References
- Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn. Reson. Med. 2009;62:656–664 doi: 10.1002/mrm.22031.
- Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn. Reson. Med. 2018;79:2306–2314 doi: 10.1002/mrm.26893.
- Mendes Pereira L, Wech T, Weng AM, et al. UTE-SENCEFUL: first results for 3D high-resolution lung ventilation imaging. Magn. Reson. Med. 2019;81:2464–2473 doi: 10.1002/mrm.27576.
- Chassagnon G, Martin C, Marini R, et al. Use of Elastic Registration in Pulmonary MRI for the Assessment of Pulmonary Fibrosis in Patients with Systemic Sclerosis. Radiology 2019;291:487–492 doi: 10.1148/radiol.2019182099.
- Jiang W, Ong F, Johnson KM, et al. Motion robust high resolution 3D free-breathing pulmonary MRI using dynamic 3D image self-navigator. Magn. Reson. Med. 2018;79:2954–2967 doi: 10.1002/mrm.26958.
- Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn. Reson. Med. 2013;70:1241–1250 doi: 10.1002/mrm.24570.
- Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016;75:775–788 doi: 10.1002/mrm.25665.
- Liu J, Spincemaille P, Codella NCF, Nguyen TD, Prince MR, Wang Y. Respiratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition. Magn. Reson. Med. 2010;63:1230–1237 doi: 10.1002/mrm.22306.
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54:2033–2044 doi: 10.1016/j.neuroimage.2010.09.025.
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41 doi: 10.1016/j.media.2007.06.004.
Figures
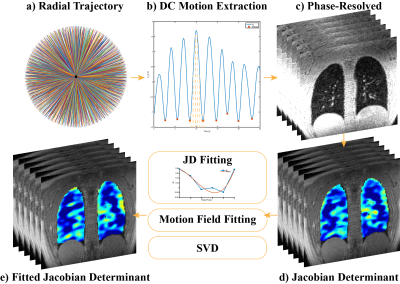
Figure 1. Pipeline for phase-resolved UTE lung MRI reconstruction and Jacobian determinant fitting. a) Acquisition trajectory with golden-angle ordering. b) Respiratory motion estimation by the filtered center of k-space and respiratory phase separation. c) Phase-resolved UTE MRI. d) The Jacobian determinant of phase-resolved UTE MRI. e) Results after Jacobian determinant curve fitting, motion field curve fitting or singular value decomposition for ventilation analysis.

Figure 2. Representative phase-resolved UTE MRI of three healthy volunteers. The left column is the end-inspiration state, the fourth column is the end-expiration state, the rest of the columns are the respiratory states in between. The dashed line suggests the reference position of the diaphragm at the end-inspiration state. Note that the diaphragm positions immediately before and after the end-expiration state are slightly different, which indicates the phase-resolved reconstruction can account for respiratory hysteresis.
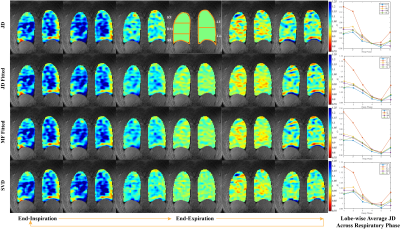
Figure 3. Ventilation analysis via Jacobian determinant (JD), Jacobian determinant fitting, motion field fitting and singular value decomposition of one healthy volunteer. JD > 1 represents expansion, JD < 1 means contraction. The first column is the end-inspiration state and the fourth column is the end-expiration state. The lobe wise average Jacobian determinant is reported on the right column.
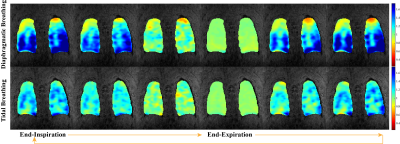
Figure 4. Ventilation maps with fitted Jacobian determinant method of one healthy volunteer under diaphragmatic breathing and tidal breathing. Note that the color bar is different from the previous figures to display the larger volume change observed in diaphragmatic breathing.